Abstract
Background
CT-P13 is the first biosimilar monoclonal antibody to infliximab. However, the antibody was tested only in rheumatoid arthritis and ankylosing spondylitis, which demonstrated equivalence to the originator in efficacy, safety, and pharmacokinetic profile. Extrapolation of its efficacy and safety to other pathologies is tenuous. Interchangeability with its originator is another unclear area.
Aim
We aimed to describe the experience of CT-P13 use in inflammatory bowel disease at a tertiary center.
Methods
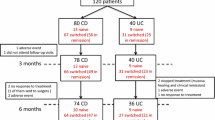
Seventeen subjects diagnosed with Crohn’s disease (CD, n = 8) or ulcerative colitis (UC, n = 9) who were administered CT-P13 from November 2012 to October 2013 at Dongguk University Ilsan Hospital were retrospectively enrolled. Medical records analyzed included patients’ characteristics, previous history of anti-tumor necrosis factor administration, response and remission to this biosimilar antibody, disease flare-up, and adverse drug reaction.
Results
Male–female ratio was 1.8. Mean age was 35.4 years (range 15–57). Mean number of CT-P13 administrations was 4.2 ± 1.9. Induction treatments were done in five UC and three CD patients. Clinical response and remission at 8 weeks were achieved in seven patients (five UC and two CD). One CD patient did not respond to CT-P13. Nine patients in maintenance with the originator were interchanged with CT-P13 (four UC and five CD patients). One UC patient experienced arthralgia and CT-P13 was discontinued. One patient experienced loss of response during the study period.
Conclusions
CT-P13 may have biosimilarity and interchangeability with its originator in inflammatory bowel disease. A large, randomized, double-blind, prospective study is needed.


Similar content being viewed by others
References
Rutgeerts P, Van Assche G, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease—seven years on. Aliment Pharmacol Ther. 2006;23:451–463.
Norum J, Koldingsnes W, Aanes T, et al. The economic burden of TNFα inhibitors and other biologic treatments in Norway. ClinicoEconomics Outcomes Res (CEOR). 2011;3:73.
World Health Organization. WHO expert committee on biological standardization. World Health Organ Tech Rep Ser. 2013;978:1–384, back cover.
Kanase SJ, Gavhane YN, Khandekar A, et al. Biosimilar: an overview. Int J Pharm Sci Res. 2013;4:2132–2144.
Schellekens H. Bioequivalence and the immunogenicity of biopharmaceuticals. Nat Rev Drug Discov. 2002;1:457–462.
Malik NN. Controlling the cost of innovative cancer therapeutics. Nat Rev Clin Oncol. 2009;6:550–552.
Generic Pharmaceutical Association. Savings achieved through the use of generic pharmaceuticals 2000–2009. http://www.gphaonline.org/sites/default/files/GPhA%20Savings%20Study%20Book%20Updated%20Web%20FINAL%20Jul23%2010_0.pdf. Accessed 12 May 2011.
Epstein MS, Ehrenpreis ED, Kulkarni PM. Biosimilars: the need, the challenge, the future: the FDA perspective. Am J Gastroenterol. 2014. doi:10.1038/ajg.2014.151.
Weise M, Bielsky M-C, De Smet K, et al. Biosimilars: what clinicians should know. Blood. 2012;120:5111–5117.
Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620.
Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–1612.
Dignass A, Van Assche G, Lindsay J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohn’s Colitis. 2010;4:28–62.
Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. Washington, DC: Food and Drug Administration; 2003.
Best WR, Becktel J, Singleton J, et al. Development of a Crohn’s disease activity index. Gastroenterology. 1976;70:439–444.
D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786.
Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti–tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Travis S, Dinesen L. Remission in trials of ulcerative colitis: what does it mean? Pract Gastroenterol. 2006;30:17.
Clark M, Colombel J-F, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21–23, 2006. Gastroenterology. 2007;133:312–339.
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med. 1997;337:1029–1036.
Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405.
Rutgeerts P. A critical assessment of new therapies in inflammatory bowel disease. J Gastroenterol Hepatol. 2002;17:S176–S185.
Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–853.
Rutgeerts P, Feagan B, Olson A, et al. A randomized placebo-controlled trial of infliximab therapy for active ulcerative colitis: act 1 trial. Gastroenterology. 2005;128:A105.
Sandborn W, Reinisch W, Rachmilewitz D, et al. Infliximab induction and maintenance therapy for ulcerative colitis: the act 2 trial. Zeitschrift für Gastroenterologie. 2005;43:V6.
Farrell RJ, Shah SA, Lodhavia PJ, et al. Clinical experience with infliximab therapy in 100 patients with Crohn’s disease. Am J Gastroenterol. 2000;95:3490–3497.
Colombel J-F, Loftus EV Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, YS., Moon, H.H., Lee, S.E. et al. Clinical Experience of the Use of CT-P13, a Biosimilar to Infliximab in Patients with Inflammatory Bowel Disease: A Case Series. Dig Dis Sci 60, 951–956 (2015). https://doi.org/10.1007/s10620-014-3392-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3392-z