Abstract
Backgrounds
Although many researchers have concentrated on the mechanism of T cell anergy resulting from over-expression of Indoleamine 2,3-dioxygenase (IDO), it remains unclear what alterations will developed in memory T cells (Tm) under over-expression of IDO.
Methods
Immunohistochemical staining for IDO expression in gastric cancer tissues (n = 50) was carried out. Tumor-infiltrating memory Tm cells were counted by flow cytometry. The association between IDO expression and the subsets of tumor infiltrating Tm are discussed.
Results
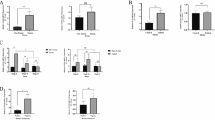
The higher IDO expressions were significantly associated with deeper invasion (P = 0.016) and higher frequencies (P = 0.038) of lymph node metastasis. The lower tumor-infiltrating CD4+Tm were significantly associated with the advanced clinical stage (P = 0.026) and lymph node metastasis (P = 0.016). The lower percentages of CD8+Tm were significantly related to undifferentiated histological type (P = 0.042) and lymph node metastasis (P = 0.037). However, the lower percentage of CD8+Tem was significantly correlated to differentiated histological type (P = 0.017), lower frequencies of lymph node metastasis (P = 0.014), and earlier clinical stage (P = 0.008). The higher IDO expression patients had significantly lower percentages of CD4+Tm (P = 0.012) and CD8+Tm (P = 0.033). Nevertheless, it was confirmed that the higher level of IDO expression correlated with higher percentages of CD8+Tm cells in univariate and multivariate analysis (P = 0.011).
Conclusion
IDO over-expression and Tm in tumor microenvironments were correlated with the disease stage and histological type of gastric cancer. Higher IDO expression was related to the lower percentage of CD4+Tm and CD8+Tm, whereas the higher level of IDO expression related with a higher percentage of CD8+Tem.



Similar content being viewed by others
References
Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15.
Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated l-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338:12–19.
Zhang R, Li H, Yu J, et al. Immunoactivative role of indoleamine 2,3dioxygenase in gastric cancer cells in vitro. Mol Med Report. 2011;4:169–173.
Yu J, Sun J, Wang SE, et al. Upregulated expression of indoleamine 2, 3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol. 2011;2011:469135.
Astigiano S, Morandi B, Costa R, et al. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia. 2005;7:390–396.
Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151.
Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372.
Li R, Wei F, Yu J, Li H, Ren X, Hao X. IDO inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Biol Ther. 2009;8:1402–1408.
Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712.
Manjunath N, Shankar P, Wan J, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878.
Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234.
Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576.
Liu Z, Dai H, Wan N, et al. Suppression of memory CD8 T cell generation and function by tryptophan catabolism. J Immunol. 2007;178:4260–4266.
Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651–658.
Smyth MJ, Trapani JA. Lymphocyte-mediated immunosurveillance of epithelial cancers? Trends Immunol. 2001;22:409–411.
Inaba T, Ino K, Kajiyama H, et al. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol. 2010;117:423–428.
Huang TT, Yen MC, Lin CC, et al. Skin delivery of short hairpin RNA of indoleamine 2,3 dioxygenase induces antitumor immunity against orthotopic and metastatic liver cancer. Cancer Sci. 2011;102:2214–2220.
Ino K, Yamamoto E, Shibata K, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310–2317.
Rauser S, Langer R, Tschernitz S, et al. High number of CD45RO+ tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I-IIA) esophageal adenocarcinoma. BMC Cancer. 2010;10:608.
Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666.
Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29:601–603.
Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366.
Hotta K, Sho M, Fujimoto K, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–1196.
Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711.
Dai H, Dai Z. The role of tryptophan catabolism in acquisition and effector function of memory T cells. Curr Opin Organ Transplant. 2008;13:31–35.
Rohde J, Heitman J, Cardenas ME. The TOR kinases link nutrient sensing to cell growth. J Biol Chem. 2001;276:9583–9586.
Narasimhan J, Staschke KA, Wek RC. Dimerization is required for activation of eIF2 kinase Gcn2 in response to diverse environmental stress conditions. J Biol Chem. 2004;279:22820–22832.
Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774.
Mondino A, Mueller DL. mTOR at the crossroads of T cell proliferation and tolerance. Semin Immunol. 2007;19:162–172.
Millan O, Jimenez O, Fortuna V, Barcelo JJ, Brunet M. Role of FK778 alone or in combination with tacrolimus or mTOR inhibitors as an immunomodulator of immunofunctions: in vitro evaluation of T cell proliferation and the expression of lymphocyte surface antigens. Int J Immunopathol Pharmacol. 2006;19:317–330.
Nam JH. Rapamycin: could it enhance vaccine efficacy? Expert Rev Vaccines. 2009;8:1535–1539.
Wiesel M, Walton S, Richter K, Oxenius A. Virus-specific CD8 T cells: activation, differentiation and memory formation. Apmis. 2009;117:356–381.
Bassett JD, Swift SL, VanSeggelen H, et al. Combined mTOR inhibition and OX40 agonism enhances CD8(+) T cell memory and protective immunity produced by recombinant adenovirus vaccines. Mol Ther. 2012;20:860–869.
Jobim PF, Pedroso TR, Werenicz A, et al. Impairment of object recognition memory by rapamycin inhibition of mTOR in the amygdala or hippocampus around the time of learning or reactivation. Behav Brain Res. 2012;228:151–158.
Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article concentrates on the impact of indoleamine 2,3-dioxygenase on infiltrating memory T cells in gastric cancer.
Rights and permissions
About this article
Cite this article
Zhang, R., Liu, H., Li, F. et al. The Correlation Between the Subsets of Tumor Infiltrating Memory T Cells and the Expression of Indoleamine 2,3-Dioxygenase in Gastric Cancer. Dig Dis Sci 58, 3494–3502 (2013). https://doi.org/10.1007/s10620-013-2837-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2837-0