Abstract
Introduction
A significant proportion of patients with Crohn’s disease (CD) lose response to antibodies directed against tumor necrosis factor α (TNF). Prior TNF-antagonist failure is associated with lower rates of response to subsequent TNF-antagonist therapy. In patients failing two anti-TNF agents, a choice exists between using a third-anti-TNF therapy or natalizumab (NAT), an α-4 integrin inhibitor. A cost-effectiveness analysis comparing these competing strategies has not been performed.
Methods
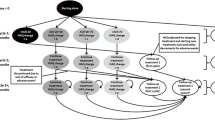
A decision analytic model was constructed to compare the performance of certolizumab pegol (CZP) versus NAT in patients with moderate to severe CD. Previously published estimates of efficacy of third-line anti-TNF therapy and NAT were used to inform the model. Costs were expressed in 2010 US dollars. A 1-year time frame was used for the analysis.
Results
In the base case estimate, use of NAT was only marginally more effective [0.71 vs. 0.70 quality adjusted life-years (QALYs)] than CZP but was expensive with an incremental cost-effectiveness ratio (ICER) of $381,678 per QALY gained. For CZP 2 months response rate of at least 24%, NAT had an ICER above the willingness-to-pay (WTP) threshold. The model was sensitive to the costs of both therapies; for all CZP costs below $2,300 per dose, NAT had higher ICER than the WTP threshold. Substituting adalimumab for CZP resulted in similar ICER estimates and thresholds for NAT use.
Conclusions
In patients with moderate to severe CD failing two TNF-antagonists, using a third TNF-antagonist therapy appears to be a cost-effective strategy without significantly compromising treatment efficacy.





Similar content being viewed by others
References
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250.
Gonzaga JE, Ananthakrishnan AN, Issa M, et al. Durability of infliximab in Crohn’s disease: a single-center experience. Inflamm Bowel Dis. 2009;15:1837–1843.
Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500.
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838.
Sandborn WJ, Abreu MT, D’Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010;8:688–695 e2.
D’Haens GR, Panaccione R, Higgins PD, et al. The London position statement of the world congress of gastroenterology on biological therapy for IBD with the European Crohn’s and colitis organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212.
Hanauer SB, Panes J, Colombel JF, Bloomfield R, Schreiber S, Sandborn WJ. Clinical trial: impact of prior infliximab therapy on the clinical response to certolizumab pegol maintenance therapy for Crohn’s disease. Aliment Pharmacol Ther. 2010;32:384–393.
Allez M, Vermeire S, Mozziconacci N, et al. The efficacy and safety of a third anti-TNF monoclonal antibody in Crohn’s disease after failure of two other anti-TNF antibodies. Aliment Pharmacol Ther. 2010;31:92–101.
Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925.
Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446.
Silverstein MD, Loftus EV, Sandborn WJ, et al. Clinical course and costs of care for Crohn’s disease: Markov model analysis of a population-based cohort. Gastroenterology. 1999;117:49–57.
Ng SC, Chan FK, Sung JJ. Review article: the role of non-biological drugs in refractory inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:417–427.
Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933.
Dubinsky MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239–2247.
2010 Drug Topics Red Book. PDR Network, LLC, 2010.
Kaplan GG, Hur C, Korzenik J, Sands BE. Infliximab dose escalation versus initiation of adalimumab for loss of response in Crohn’s disease: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2007;26:1509–1520.
Malone DC, Waters HC, Van Den Bos J, Popp J, Draaghtel K, Rahman MI. A claims-based Markov model for Crohn’s disease. Aliment Pharmacol Ther. 2010;32:448–458.
Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Mak. 2002;22:417–430.
Sandborn WJ, Schreiber S, Hanauer SB, Colombel JF, Bloomfield R, Lichtenstein GR. Reinduction with certolizumab pegol in patients with relapsed Crohn’s disease: results from the PRECiSE 4 Study. Clin Gastroenterol Hepatol. 2010;8:696–702 e1.
Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355–366.
Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–1239.
Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol. 2010;105:1574–1582.
Kugathasan S, Werlin SL, Martinez A, Rivera MT, Heikenen JB, Binion DG. Prolonged duration of response to infliximab in early but not late pediatric Crohn’s disease. Am J Gastroenterol. 2000;95:3189–3194.
Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009;58:1612–1619.
Arijs I, Quintens R, Lommel LV, et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010;16:2090–2098.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ananthakrishnan, A.N., Hur, C. & Korzenik, J.R. Certolizumab Pegol Compared to Natalizumab in Patients with Moderate to Severe Crohn’s Disease: Results of a Decision Analysis. Dig Dis Sci 57, 472–480 (2012). https://doi.org/10.1007/s10620-011-1896-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-011-1896-3