Abstract
Symptoms of choledochal cysts are caused by protein plugs. We performed proteomic analysis of protein plugs to elucidate formation mechanism. Protein plugs were obtained from three pediatric patients with choledochal cyst. Proteins were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Gel bands common to the samples were excised for mass spectrometry. Mass spectra were compared with the NCBI database for protein identification. Gel bands of protein plug samples were predominant at 14 kilodaltons (kDa), followed by 29 kDa. Four other thin bands were common to the plug samples. Four bands (including 14 and 29 kDa) were identified as lithostathine, and one band as serum albumin. Plugs consisted mostly of lithostathine, a protein secreted by pancreatic acinar cells into pancreatic juice. The mechanism involves trypsinogen and lithostathine regurgitating into the cyst through an aberrant union of pancreaticobiliary ducts. Activated trypsin cleaves soluble lithostathine into insoluble forms that aggregate to form plugs.
Similar content being viewed by others
Introduction
Choledochal cysts are not frequently encountered in Western countries but are common in Asia among all age groups. Five types have been documented, but most cases are classified in one of two types: congenital dilation of the common bile duct with or without dilated intrahepatic ducts (type I and type IV-A according to Todani's classification) [1–3]. The two types may comprise one entity that always accompanies another anomaly, namely, pancreaticobiliary maljunction, which is defined as the aberrant union of the pancreatic and biliary ducts located outside the duodenal wall [2–5]. Because Oddi's sphincter is not involved in the union, pancreaticobiliary maljunction causes two-way regurgitation of bile and pancreatic juice, damaging these systems [2, 4–6]. During childhood, it produces characteristically intermittent symptoms including abdominal pain, vomiting, and jaundice. In adults, it causes malignant transformation in the gallbladder and cysts [2, 3, 5].
Treatment of choledochal cyst/pancreaticobiliary maljunction is aimed at preventing symptoms and biliary carcinogenesis. Drainage procedures (e.g., cystenterostomy), once common, have been abandoned because of the high rate of complications by choledocholithiasis and biliary malignancy. At present, surgical excision of the extrahepatic bile duct with Roux-Y hepaticojejunostomy is recommended to rectify pancreaticobiliary maljunction [2, 3]. However, cyst excision has shown late complications. Intrahepatic calculi form at a rate of 7% to 8% after cyst excision [7]. These calculi are brown pigment stones composed mainly of calcium bilirubinate. Bile stasis and bacterial infection are important factors in the formation of these postoperative gallstones as well as other brown pigment stones [8]. Biliary reconstruction followed by cyst excision neutralizes the defense mechanism of Oddi's sphincter against bacteria, and causes bile infection [7]. Bile stasis is caused by anastomotic stricture of hepaticoenterostomy or congenital stenosis and dilatation of intrahepatic bile ducts [2, 9, 10]. Carcinogenesis in the residual bile ducts is also increasingly reported [11–14]. Kobayashi et al. encountered three such patients after 47 choledochal cyst excisions, and concluded by the person-year statistical analysis that the incidence of bile duct carcinoma after cyst excision is still 121.5 times higher than in the general population [12]. This history of treatment indicates that research should aim for nonsurgical management.
Preoperative cholangiopancreatography demonstrated protein plugs as filling defects in the common channel (upper left; Patient 2) and in the common hepatic duct (lower right; Patient 3). Operative photograph shows protein plugs removed with a surgical spoon (upper right; Patient 2). Macroscopic photograph of obtained protein plugs shows yellowish-white fragile granular materials (lower left; Patient 2)
Previous studies of pancreaticobiliary maljunction/choledochal cyst focused on carcinogenesis, and little attention has been paid to symptomatology. We previously reported that protein plugs, generally considered rare, are found as frequently as in 40% of cases, and must be a causative agent of symptoms [5]. Protein plugs are compacted in the common channel, obstruct the bile and pancreatic ducts, and increase intraductal pressure, resulting in symptoms. Most plugs are fragile and disappear spontaneously, which explains the self-limiting nature of the symptoms. This transient nature of the plugs may be the reason why patients with choledochal cysts present later in life. Some plugs do not disappear and exacerbate symptoms, requiring external biliary drainage for symptomatic relief [5]. In rare cases, plugs become firm and cause a significantly increased intraductal pressure, leading to perforation of the choledochal cyst [15]. Characteristically intermittent symptoms may be due to repeated plug formation. In patients with pancreaticobiliary maljunction not complicated by choledochal cysts, protein plugs are also a causative agent of symptoms [16]. Stopping protein plugs from forming is expected to prevent symptoms. To understand the formation mechanism, we performed proteomic analysis of protein plugs from patients with pancreaticobiliary maljunction/choledochal cysts.
Materials and methods
Materials
Samples from patients were collected in accordance with the approved human subject guidelines of Nagoya University Graduate School of Medicine. Between October 2004 and May 2005, we encountered four pediatric patients with choledochal cysts. All patients had symptoms of abdominal pain with vomiting or jaundice, and underwent surgery soon after symptoms subsided. From three of the four patients, protein plugs were obtained during surgery. In a 1-year-old girl, protein plugs were incidentally found in the common bile duct at the time of cyst excision (Patient 1). In a 3-year-old girl, preoperative endoscopic retrograde cholangiopancreatography demonstrated filling defects in the common channel, and protein plugs were removed from the common channel during surgery (Patient 2; Fig. 1). In another 3-year-old girl, preoperative magnetic resonance cholangiopancreatography demonstrated protein plugs as filling defects in the common hepatic duct, and the plugs were removed from the common bile duct (Patient 3; Fig. 1). Obtained protein plugs were washed with distilled water and centrifuged, and this was repeated four times for the removal of bile, pancreatic juice, and blood. Bile samples were obtained from the gallbladders of the three patients with choledochal cysts and three other children (4- and 13-year-old girls and a 4-year-old boy) without pancreatobiliary diseases as controls.
Infrared absorption spectrometry of protein plugs
To confirm that the obtained specimens consisted of proteins, the infrared absorption spectra of protein plug samples were measured using a VIR-9500 Fourier transform infrared spectrophotometer (JASCO, Hachioji, Japan). Materials were dried, crushed into a powder, mixed with powdered potassium bromide, and analyzed.
Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Each protein plug sample (0.5 μl) and 5 μl of each bile sample were precipitated by the addition of 10% (wt/vol) trichloroacetic acid. The pellets were resuspended in SDS sample buffer containing a reducing agent and heated for 5 min at 95°C. Proteins were separated by SDS-PAGE on a 15% polyacrylamide gel. The gel was stained with SYPRO Ruby (Invitrogen Japan, Tokyo).
SDS-PAGE of bile and protein plug samples. Protein plug samples had a predominant band at 14 kDa. Arrows indicate six bands common to the protein plug samples, which were analyzed by mass spectrometry. Choledochal cyst bile contains more bands than normal bile, reflecting regurgitated pancreatic proteins besides bile proteins. Mr, molecular weight markers; C1-3, normal controls without pancreaticobiliary diseases; P1–P3, patients with pancreaticobiliary maljunction/choledochal cyst
Protein identification by peptide mass fingerprinting
Bands common to the plug samples were excised from the SDS-PAGE gel. The excised gel pieces were digested with trypsin as described by Shevchenko et al. [17]. Peptides were desalted with a reversed-phase ZipTip C18 (Millipore, Bedford, MA, USA), eluted with the ionization matrix solution of α-cyano-4-hydroxycinnamic acid, and spotted on the sample plate for mass spectrometry. Peptides were analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry in reflector mode using a Voyager-DE STR (Applied Biosystems, USA). Mass spectra were compared with the nonredundant NCBI database using the MS-Fit search engine (http://prospector.ucsf.edu/). A mass tolerance of 20 ppm was used. Peptide amino-terminal glutamine to pyroglutamic acid, oxidation of methionine, protein amino-terminus acetylated, and acrylamide modified cysteine were considered as possible modifications. Proteins with a MOWSE score of >1000 and hits by seven or more peptides were “identified” by peptide mass fingerprinting. In cases below but near this criterion, the spectral patterns and peak intensities of hit peptides were considered for identification.
Results
Infrared spectrum
Infrared spectra of the three materials of protein plugs showed almost the same pattern as that of standard protein (Fig. 2). The spectra had two major absorption bands, at about 1650 and 1550–1500 cm−1, which were characteristic of a peptide band (Fig. 2; arrows).
Matrix-assisted laser desorption ionization time-of-flight spectra of each band common to the three protein plug samples. The 14- and 29-kDa bands of Patient 1 and the others of Patient 3 were used for mass spectrometry. Peaks marked by arrows are peptide signals assigned to the corresponding peptides from lithostathine 1 α precursor. Spectra of 6-, 10-, and 27-kDa bands showed a similar modification of peptide signals adding masses in multiples of 16
SDS-PAGE
Protein plugs had different band patterns than the bile samples (Fig. 3). The band pattern was similar among protein plug samples. The band at 14 kilodaltons (kDa) was predominant, followed by that at 29 kDa. Four other thin bands were common to protein plug samples (6, 10, 27, and 66 kDa). Among bile samples, the band pattern of choledochal cyst samples was different from that of normal bile. Bile from choledochal cysts had many different bands besides those seen in normal bile.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry and peptide mass fingerprinting
Figure 4 shows mass spectra of the six bands common to the protein plug samples. Peptide mass fingerprinting identified that three bands (10, 14, and 29 kDa) were proteins derived from the same protein, lithostathine 1 α precursor (UniProt/Swiss-Prot accession number, P05451) (Fig. 5). The 66-kDa band was identified as serum albumin. Although the 6- and 27-kDa bands did not reach the criteria for identification, four peptide masses of the 6-kDa band and six peptide masses of the 27-kDa band matched those of the lithostathine1 α precursor, with MOWSE scores of 133 and 820, respectively. Among these, the 27-kDa band was judged as lithostathine, since the spectrum was similar to those of the 10-, 14-, and 29-kDa bands, and peaks of the six hit peptide masses were intense enough. The spectra of the 6-, 10-, and 27-kDa bands had similar modified signals of hit peptide masses plus multiples of 16 m/z. The hit peptides of the 6-, 10-, and 27-kDa bands did not cover carboxyl-terminal sequences of lithostathine compared to those of the 14- and 29-kDa bands.
Complete amino acid sequence of lithostathine 1 α precursor. Peptides of the 14-kDa band, matched by peptide mass fingerprinting, are underlined in boldface, with a sequence coverage of 54%. The first 1–22 is a signal peptide (half-tone meshed in italics), and the remaining 23–166 represents a secretory form of lithostathine (S2). The arrow indicates the trypsin cleavage site (Arg–Ile) of S2, and the carboxyl-terminal 133 amino acids comprise insoluble lithostathine, S1. The matched peptides exist only within the S1 sequence
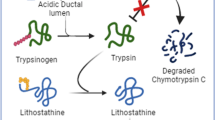
Proposed mechanism of protein plug formation in choledochal cysts. The pancreas secretes lithostathine (LIT) S2 and trypsinogen, which are regurgitated through an abnormal union into the biliary tract. Trypsinogen is activated into trypsin by an unknown mechanism. Activated trypsin cleaves S2 into an undecapeptide and S1. S1 monomers assemble into a tetramer, each of which assembles longitudinally into a protofibril. LIT fibrils aggregate into a protein plug, which clogs the common channel and causes symptoms
Discussion
This study demonstrated that protein plugs formed in choledochal cysts consist of proteins, most of which are lithostathine. Human lithostathine is encoded by REG 1A (regenerating gene) as a 166-residue preprotein, with a 22-residue amino-terminal signal sequence [18–20] (Fig. 5). Pancreatic acinar cells secrete lithostathine as a soluble 144-amino acid glycoprotein (16 kDa; called lithostathine S2) into pancreatic juice, which accounts for 5% to 10% of the secreted proteins [20–22]. Lithostathine has many other names (pancreatic stone protein, pancreatic thread protein, islet of Langerhans regenerating protein, regenerating protein 1 α, islet cell regenerating factor), reflecting its discovery history and controversial functions [20]. Lithostathine is the only protein secreted from the pancreas that has no known digestive or hormonal activity [23]. The pathological significance involves its highly aggregative properties [24]. Lithostathine S2, a soluble secretory form, is highly susceptible to trypsin cleavage at the arginine–isoleucine bond in positions 11–12 (Fig. 5; arrow). Cleaved S2 produces an amino-terminal undecapeptide and a 14-kDa carboxyl-terminus with 133 amino acids (called S1 or pancreatic thread protein). Lithostathine S1 is insoluble, and readily polymerizes at physiologic pH. Dimers of S1 evolve by lateral hydrophobic interactions into tetramers, which self-assemble by longitudinal electrostatic interactions into protofibrils, resulting in a network of fibrillar aggregates [18, 24–26]. These fibrillar structures are very resistant to the wide spectrum of proteases contained in pancreatic juice [25]. Lithostathine fibrils are found in precipitates, protein plugs, and radiolucent stones in the pancreatic ducts of patients with chronic pancreatitis [27–29]. Lithostathine deposits are also found in neurodegenerative diseases such as Alzheimer's disease and Creutzfeldt-Jakob disease, and may be involved in amyloidosis and tangle formation by allowing heterogeneous precipitation of other proteins [26, 30–32].
The present data showed that the major component of the protein plug is 14-kDa lithostathine S1, the trypsin-cleaved form. The secondary major component at 29 kDa represents a dimer of S1. These results clearly indicate that the protein plugs are mainly made of self-assembled lithostathine fibrils after trypsin cleavage. Before this study, we had expected detection of other substances that can aggregate proteins, such as mucin core proteins and lactoferrin [5, 33]. However, minor components detected at 6, 10, and 27 kDa were also identified or estimated as lithostathines, except for the 66-kDa serum albumin. These three lithostathines are supposed to be proteolytic and oxidized forms of both S1 and its dimer, because their hit peptides did not cover the carboxyl-terminal sequence of lithostathine, and because the three mass spectra showed the same modified peptide signals of hit peptide masses plus multiples of 16. It is unknown whether this change of lithostathines has a pathological meaning or is only caused due to experimental procedures. The 66-kDa albumin may represent contamination, because albumin is contained in blood, bile, and pancreatic juice, which cannot be removed completely from surgical samples [34–36]. However, albumin possesses a good binding capacity for chemically diverse molecules, and is reported to consist of clogging material that occludes pancreatic stents placed in the pancreatic ducts of patients with chronic pancreatitis during endoscopic management [37–39]. Albumin seems to be a minor but true component of protein plugs, binding to lithostathines. It is concluded that protein plugs in choledochal cysts are made of trypsin-cleaved lithostathines, containing a small quantity of albumin and disintegrated lithostathines.
The present SDS-PAGE results showed bile samples from choledochal cyst patients contain a greater variety of proteins besides original bile proteins, which must be derived from pancreatic proteins regurgitated through an abnormal union. Recently, we have demonstrated that pancreatic enzymes and lithostathine regurgitate into the biliary tract of patients with choledochal cysts, and that trypsin is activated to a varying degree [40]. Therefore, the following mechanism is considered most likely (Fig. 6): pancreaticobiliary maljunction causes regurgitation of pancreatic juice into the biliary tract and activates pancreatic proenzymes through an unknown mechanism. Regurgitated pancreatic juice also contains soluble lithostathine, S2. Activated trypsin cleaves S2 into insoluble S1, which then aggregates into lithostathine protofibrils. Lithostathine protofibrils grow to fibrils and form protein plugs. Protein plugs flow down and are stuck in the long common channel. Plugs obstruct the common channel by a ball valve mechanism, and provoke increased intraductal pressure of pancreaticobiliary ducts, resulting in characteristic symptoms.
This understanding of the protein plug formation mechanism is the first step toward nonsurgical treatment for choledochal cyst/pancreaticobiliary maljunction. Agents that inhibit the association of individual lithostathine fibrils can be a drug for the relief or prevention of symptoms, by dissolving plugs or preventing their formation. Laurine et al. have shown that 6,6′-bibenzothiazolyl-2,2′-diamine, a derivative of benzothiazoles, can dissociate lithostathine bundles into individual protofilaments in vitro [31]. Another benzothiazole or riluzole (2-amino-6-trifluoromethoxy-benzothiazole) has been used for neurodegenerative diseases and found to be effective in some disorders [41–43]. Benzothiazoles were found to disrupt insoluble protein aggregates of Huntington's disease [42]. Benzothiazoles are candidates for a new therapeutic approach for choledochal cysts as well as neurodegenerative disorders. Of course, biliary carcinogenesis is another more serious problem in treatment for choledochal cyst/pancreaticobiliary maljunction. This issue is also beginning to be approached by chemoprevention. Tsuchida et al. recently reported the efficacy of a cyclooxygenase-2 inhibitor in suppressing carcinogenesis in a hamster model of pancreaticobiliary maljunction [44]. Research to identify nonsurgical therapy from both aspects of carcinogenesis and symptomatology has just begun.
In conclusion, protein plugs complicating choledochal cyst/pancreaticobiliary maljunction consisted mostly of lithostathine, a protein secreted by pancreatic acinar cells into pancreatic juice. The presumptive mechanism involves trypsinogen and lithostathine regurgitating into the cyst through an aberrant union of the bile and pancreatic ducts. Activated trypsin cleaves soluble lithostathine into insoluble forms that aggregate to form plugs. This understanding of the protein plug formation mechanism is expected to lead to nonsurgical methods to dissolve plugs or prevent their formation in the future.
References
Todani T, Watanabe Y, Naruse M, Tabuchi K, Okajima K (1977) Congenital bile duct cysts: classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg 134:263–269
Todani T (1997) Congenital choledochal dilatation: classification, clinical features, and long-term results. J Hepatobil Pancreat Surg 4:276–282
Visser BC, Suh I, Way LW, Kang SM (2004) Congenital choledochal cysts in adults. Arch Surg 139:855–862
Japanese Study Group on Pancreaticobiliary Maljunction (JSPBM), Committee of JSPBM for Diagnostic Criteria (1994) Diagnostic criteria of pancreaticobiliary maljunction. J Hepatobil Pancreat Surg 1:219–221
Kaneko K, Ando H, Ito T, Watanabe Y, Seo T, Harada T, Ito F (1997) Protein plugs cause symptoms in patients with choledochal cysts. Am J Gastroenterol 92:1018–1021
Kaneko K, Ando H, Ito T, Kasai K, Watanabe Y, Seo T (1996) Increased cell proliferation and transforming growth factor-alpha (TGF alpha) in the gall-bladder epithelium of patients with pancreaticobiliary maljunction. Pathol Int 46:253–260
Kaneko K, Ando H, Seo T, Ono Y, Ochiai K, Ogura Y (2005) Bile infection contributes to intrahepatic calculi formation after excision of choledochal cysts. Pediatr Surg Int 21:8–11
Maki T (1966) Pathogenesis of calcium bilirubinate stone: role of E. coli, b-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg 164:90–100
Ando H, Ito T, Kaneko K, Seo T, Ito F (1996) Intrahepatic bile duct stenosis causing intrahepatic calculi formation following excision of a choledochal cyst. J Am Coll Surg 183:56–60
Uno K, Tsuchida Y, Kawarasaki H, Ohmiya H, Honna T (1996) Development of intrahepatic cholelithiasis long after primary excision of choledochal cysts. J Am Coll Surg 183:583–588
Watanabe Y, Toki A, Todani T (1999) Bile duct cancer developed after cyst excision for choledochal cyst. J Hepatobil Pancreat Surg 6:207–212
Kobayashi S, Asano T, Yamasaki M, Kenmochi T, Nakagohri T, Ochiai T (1999) Risk of bile duct carcinogenesis after excision of extrahepatic bile ducts in pancreaticobiliary maljunction. Surgery 126:939–944
Goto N, Yasuda I, Uematsu T, Kanemura N, Takai S, Ando K, Kato T, Osada S, Takao H, Saji S, Shimokawa K, Moriwaki H (2001) Intrahepatic cholangiocarcinoma arising 10 years after the excision of congenital extrahepatic biliary dilation. J Gastroenterol 36:856–862
Tsuchida A, Kasuya K, Endo M, Saito H, Inoue K, Nagae I, Aoki T, Koyanagi Y (2003) High risk of bile duct carcinogenesis after primary resection of a congenital biliary dilatation. Oncol Rep 10:1183–1187
Ando H, Ito T, Watanabe Y, Seo T, Kaneko K, Nagaya M (1995) Spontaneous perforation of choledochal cyst. J Am Coll Surg 181:125–128
Ando H, Ito T, Nagaya M, Watanabe Y, Seo T, Kaneko K (1995) Pancreaticobiliary maljunction without choledochal cysts in infants and children: clinical features and surgical therapy. J Pediatr Surg 30:1658–1662
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68:850–858
Giorgi D, Bernard JP, Rouquier S, Iovanna J, Sarles H, Dagorn JC (1989) Secretory pancreatic stone protein messenger RNA. Nucleotide sequence and expression in chronic calcifying pancreatitis. J Clin Invest 84:100–106
Watanabe T, Yonekura H, Terazono K, Yamamoto H, Okamoto H (1990) Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues: the reg protien, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. J Biol Chem 265:7432–7439
Patard L, Lallemand JY, Stoven V (2003) An insight into the role of human pancreatic lithostathine. JOP 4:92–103
Provansal-Cheylan M, Mariani A, Bernard JP, Sarles H, Dupuy P (1989) Pancreatic stone protein: quantification in pancreatic juice by enzyme-linked immunosorbent assay and comparison with other methods. Pancreas 4:680–689
Schmiegel W, Burchert M, Kalthoff H, Roeder C, Butzow G, Grimm H, Kremer B, Soehendra N, Schreiber HW, Thiele HG, Greten H (1990) Immunochemical characterization and quantitative distribution of pancreatic stone protein in sera and pancreatic secretions in pancreatic disorders. Gastroenterology 99:1421–1430
Lee BI, Mustafi D, Cho W, Nakagawa Y (2003) Characterization of calcium binding properties of lithostathine. J Biol Inorg Chem 8:341–347
Gregoire C, Marco S, Thimonier J, Duplan L, Laurine E, Chauvin JP, Michel B, Peyrot V, Verdier JM (2001) Three-dimensional structure of the lithostathine protofibril, a protein involved in Alzheimer's disease. EMBO J 20:3313–3321
Graf R, Schiesser M, Scheele GA, Marquardt K, Frick TW, Ammann RW, Bimmler D (2001) A family of 16-kDa pancreatic secretory stress proteins form highly organized fibrillar structures upon tryptic activation. J Biol Chem 276:21028–21038
Iovanna JL, Dagorn JC (2005) The multifunctional family of secreted proteins containing a C-type lectin-like domain linked to a short N-terminal peptide. Biochim Biophys Acta 1723:8–18
Guy O, Robles-Diaz G, Adrich Z, Sahel J, Sarles H (1983) Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology 84:102–107
Multigner L, Daudon M, Montalto G, De Caro A, Etienne JP, Sarles H (1986) Radiolucent pancreatic stones. N Engl J Med 314:248
Mariani A, Bernard JP, Provansal-Cheylan M, Nitsche S, Sarles H (1991) Differnces of pancreatic stone morphology and content in patients with pancreatic lithiasis. Dig Dis Sci 36:1509–1516
Ozturk M, de la Monte SM, Gross J, Wands JR (1989) Elevated leveld of an exocrine pancreatic secretory protein in Alzheimer disease brain. Proc Natl Acad Sci USA 86:419–423
Laurine E, Gregoire C, Fandrich M, Engemann S, Marchal S, Thion L, Mohr M, Monsarrat B, Michel B, Dobson CM, Wanker E, Erard M, Verdier JM (2003) Lithostathine quadruple-helical filaments form proteinase K-resistant deposits in Creutzfeldt-Jakob disease. J Bio Chem 51:51770–51778
Duplan L, Michel B, Boucraut J, Barthellemy S, Desplat-Jego S, Marin V, Gambarelli D, Bernard D, Berthezene P, Alescio-Lautier B, Verdier JM (2001) Lithostathine and pancreatitis-associated protein are involved in the very early stages of Alzheimer's disease. Neurobiol Aging 22:79–88
Kaneko K, Ando H, Watanabe Y, Seo T, Harada T (2000) Pathologic changes in the common bile duct of an experimental model with pancreaticobiliary maljunction. Pediatr Surg Int 16:26–28
Kristiansen TZ, Bunkenborg J, Gronborg M, Molina H, Thuluvath PJ, Argani P, Goggins MG, Maitra A, Pandey A (2004) A proteomic analysis of human bile. Mol Cell Proteomics 3:715–728
Zhou H, Chen B, Li RX, Sheng QH, Li SJ, Zhang L, Li L, Xia QC, Wang HY, Zeng R (2005) Large-scale identification of human biliary proteins from a cholesterol stone patient using a proteomic approach. Rapid Commun Mass Spectrom 19;3569–3578
Grønborg M, Bunkenborg J, Kristiansen TZ, Jensen ON, Yeo CJ, Hruban RH, Maitra A, Goggins MG, Pandey A (2004) Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res 3:1042–1055
He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358:209–215
Smits ME, Groen AK, Mok KS, van Marle J, Tytgat GN, Huibregtse K (1997) Analysis of occluded pancreatic stents and juices in patients with chronic pancreatitis. Gastrointest Endosc 45:52–58
Farnbacher MJ, Voll RE, Faissner R, Wehler M, Hahn EG, Lohr M, Schneider HT (2005) Composition of clogging material in pancreatic endoprostheses. Gastrointest Endosc 61:862–866
Ochiai K, Kaneko K, Kitagawa M, Ando H, Hayakawa T (2004) Activated pancreatic enzyme and pancreatic stone protein (PSP/reg) in bile of patients with pancreaticobiliary maljunction/choledochal cyst. Dig Dis Sci 49:1953–1956
Bensimon G, Lacomblez L, Meininger V (1994) A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med 330:585–591
Heiser V, Engemann S, Brocker W, Dunkel I, Boeddrich A, Waelter S, Nordhoff E, Lurz R, Schugardt N, Rautenberg S, Herhaus C, Barnickel G, Bottcher H, Lehrach H, Wanker EE (2002) Identification of benzothiazoles as potential polyglutamine aggregation inhibitors of Huntington's disease by using an automated filter retardation assay. Proc Natl Acad Sci USA 99:16400–16406
Huntington Study Group (2003) Dosage effects of riluzole in Huntington's disease. A multicenter placebo-controlled study. Neurology 61:1551–1556
Tsuchida A, Itoi T, Kasuya K, Endo M, Katsumata K, Aoki T, Suzuki M, Aoki T (2005) Inhibitory effect of meloxicam, a cyclooxygenase-2 inhibitor, on N-nitrosobis (2-oxopropyl) amine induced biliary carcinogenesis in Syrian hamsters. Carcinogenesis 26:1922–1928
Acknowledgments
This work has been supported by a grant-in-aid (17591860) for Scientific Research from the Ministry of Education, Science, and Culture, Japan. We thank Kenji Yamamoto, Ikuko Sagawa, and Satoru Kuboi (APRO Life Science Institute, Naruto, Japan) for peptide mass fingerprinting analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaneko, K., Ando, H., Seo, T. et al. Proteomic Analysis of Protein Plugs: Causative Agent of Symptoms in Patients with Choledochal Cyst. Dig Dis Sci 52, 1979–1986 (2007). https://doi.org/10.1007/s10620-006-9398-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9398-4