Abstract
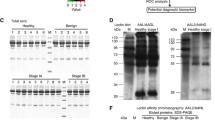
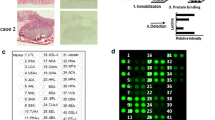
The aim of this study was to evaluate the diagnostic utility of lectin microarrays in pleural effusions of patients with lung cancer. A lectin microarray, LTL, PSA, LCA, UEA-1, AAL, MAL-I, MAL-II, SNA, WGA, ECL, DSA, STL, SWGA, HPA, ConA, GNA, HHL, BPL, EEL, Jacalin, WFA, ACL, MPL, DBA, SBA, was used to determine the glycoprotein profile of cells in pleural effusions from patients with lung cancer (54 cases), and with benign lung disease (54 cases). The A549 cell line, used as an experimental control, was positive for AAL, MAL-I, WGA, STL, Jacalin and ACL binding. Adenocarcinoma cells in pleural effusions were positive for ECL, DSA, AAL, MAL-I, WGA, STL, Jacalin, and ACL binding. AAL, WGA, and ACL positive binding was the most common, found in 54, 48, and 38 samples, respectively. ECL and DSA binding was positive in only 4 samples. In comparison, reactive mesothelial cells displayed positive binding for all markers in the microarray panel. SNA and AAL positive binding was detected in the majority of samples; 50/54 and 48/54 samples, respectively. Positive binding of DBA, MAL-II and EEL was present in only 2, 4 and 4 samples, respectively. SNA binding had the highest sensitivity (92.6 %), specificity (100 %), and accuracy (96.3 %). SNA may be used as a biomarker to distinguish reactive mesothelial cells from adenocarcinoma cells. The lectin microarrays proved able to distinguish carcinoma cells from reactive mesothelial cells in pleural effusions.



Similar content being viewed by others
References
Afify AM, Al-Khafaji BM, Paulino AF, Davila RM (2002) Diagnostic use of muscle markers in the cytologic evaluation of serous fluids. Appl Immunohistochem Mol Morphol 10:178–182
Afify AM, Stern R, Michael CW (2005) Differentiation of mesothelioma from adenocarcinoma in serous effusions: the role of hyaluronic acid and CD44 localization. Diagn Cytopathol 32:145–150
Bassarova AV, Nesland JM, Davidson B (2006) D2-40 is not a specific marker for cells of mesothelial origin in serous effusions. Am J Surg Pathol 30:878–882
Brooks SA, Leathem AJ (1991) Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet 338:71–74
Dennis JW, Granovsky M, Warren CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1473:21–34
Gabius HJ, SiebertHC AndreS, Jimenez-Barbero J, Rudiger H (2004) Chemical biology of the sugar code. ChembioChem 5:740–764
Ghayumi SM, Mehrabi S, Doroudchi M, Ghaderi A (2005) Diagnostic value of tumor markers for differentiating malignant and benign pleural effusions of Iranian patients. Pathol Oncol Res 11:236–241
Hakomori S (2002) Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA 99:10231–10233
Haltiwanger RS, Lowe JB (2004) Role of glycosylation in development. Annu Rev Biochem 73:491–537
Jiang B, Wu GP, Zhao YJ, Wang SC (2008) Transcription expression and clinical significance of TTF-1 mRNA in pleural effusion of patients with lung cancer. Diagn Cytopathol 36:849–854
Lehle L, Strahl S, Tanner W (2006) Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl 45:6802–6818
Muramatsu T (2002) Development. Carbohydrate recognition in spermatogenesis. Science 295:53–54
Politi E, Kandaraki C, Apostolopoulou C, Kyritsi T, Koutselini H (2005) Immunocytochemical panel for distinguishing between carcinoma and reactive mesothelial cells in body cavity fluids. Diagn Cytopathol 32:151–155
Queiroz C, Barral-Netto M, Bacchi CE (2001) Characterizing subpopulations of neoplastic cells in serous effusions. The role of immunocytochemistry. Acta Cytol 45:18–22
Rosenfeld R, Bangio H, Gerwig GJ, Rosenberg R, Aloni R, Cohen Y, Amor Y, Plaschkes I, Kamerling JP, Maya RB (2007) A lectin array-based methodology for the analysis of protein glycosylation. J Biochem Biophys Meth 70:415–426
Sun Y, Wu GP, Fang CQ, Liu SL (2009) Diagnostic utility of MOC-31, HBME-1 and MOC-31mRNA in distinguishing between carcinoma cells and reactive mesothelial cells in pleural effusions. Acta Cytol 53:619–624
Tao SC, Li Y, Zhou J, Qian J, Schnaar RL, Zhang Y, Goldstein IJ, Zhu H, Schneck JP (2008) Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology 18:761–769
Tateno H, Uchiyama N, Kuno A, Togayachi A, Sato T, Narimatsu H, Hirabayashi J (2007) A novel strategy for mammalian cell surface glycome profiling using lectin microarray. Glycobiology 17:1138–1146
Van Dyken SJ, Green RS, Marth JD (2007) Structural and mechanistic features of protein O glycosylation linked to CD8 + T-cell apoptosis. Mol Cell Biol 27:1096–1111
Wearne KA, Winter HC, O’Shea K, Goldstein IJ (2006) Use of lectins for probing differentiated human embryonic stem cells for carbohydrates. Glycobiology 16:981–990
Wu GP, Zhang SS, Fang CQ, Liu SL, Wang EH (2008) Immunocytochemical panel for distinguishing carcinoma cells from reactive mesothelial cells in pleural effusions. Cytopathology 19:212–217
Zhou WB, Bai M, Jin Y (2009) Diagnostic value of vascular endothelial growth factor and endostatin in malignant pleural effusions. Int J Tuberc Lung Dis 13:381–386
Zhu H, Klemic JF, Chang S, Bertone P, Casamayor A, Klemic KG, Smith D, Gerstein M, Reed MA, Snyder M (2000) Analysis of yeast protein kinases using protein chips. Nat Genet 26:283–289
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (to Guang-Ping Wu, Grant No.81171650 and to Qun He, Grant No.20672144).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, YQ., He, Q., Zhao, YJ. et al. Lectin microarrays differentiate carcinoma cells from reactive mesothelial cells in pleural effusions. Cytotechnology 65, 355–362 (2013). https://doi.org/10.1007/s10616-012-9474-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-012-9474-x