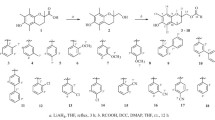
Three types of trolox dimers (bis-trolox derivatives), two of which were pairs of trolox molecules bound symmetrically through an ethylenediamine linker with covalent or ionic bonds and one of which was an asymmetric ionic–covalent conjugate, were synthesized. The water-solubility and antioxidant activity were measured for the synthesized compounds.
Similar content being viewed by others
References
J. Lee, H.-N. Kim, D. Yang, K. Jung, H.-M. Kim, H.-H. Kim, H. Ha, and Z. H. Lee, J. Biol. Chem., 284 (20), 13725 (2009); J. A. Hyatt, Synth. Commun., 38, 8 (2008); K. Nakagawa-Goto, K. Yamada, S. Nakamura, T.-H. Chen, P.-C. Chiang, K. F. Bastow, S.-C. Wang, B. Spohn, M.-C. Hung, F.-Y. Lee, F.-C. Lee, and K.-H. Lee, Bioorg. Med. Chem. Lett., 17, 5204 (2007).
S. L. Stvolinsky, E. R. Bulygina, T. N. Fedorova, K. Meguro, T. Sato, O. V. Tyulina, H. Abe, and A. A. Boldyrev, Cell. Mol. Neurobiol., 30, 395 (2010); Y. Catel, F. Aladedunye, and R. Przybylski, J. Agric. Food Chem., 58, 11081 (2010); V. R. Khairullina, A. Ya. Gerchikov, A. B. Safarova, R. R. Khalitova, A. Yu. Spivak, E. R. Shakurova, and V. N. Odinokov, Kin. Catal., 52 (2), 186 (2011); S. Dikalov and D. G. Harrison, in: Antioxidants and Cardiovascular Disease, 2nd Ed., M. G. Bourassa and J.-C. Tardif (eds.), Springer, 2006, pp. 167–194.
J.-M. Contreras and W. Sippl, in: The Practice of Medicinal Chemistry, 3rd Ed., C. G. Wermuth (ed.), Elsevier, 2008, pp. 380–414.
S. Stvolinsky, K. Toropova, M. Gordeeva, V. Kazey, T. Sato, K. Meguro, and A. Boldyrev, Amino Acids, 43, 165 (2012); S. M. Ahn, H. S. Rho, H. S. Baek, Y. H. Joo, Y. D. Hong, S. S. Shin, Y. Park, and S. N. Park, Bioorg. Med. Chem. Lett., 21, 7466 (2011); K. Shimizu, R. Kondo, K. Sakai, N. Takeda, T. Nagahata, and T. Oniki, Lipids, 36 (12), 1321 (2001); A. Yu. Spivak, R. R. Mufazzalova, E. R. Shakurova, and V. N. Odinokov, Zh. Org. Khim., 46 (9), 1355 (2010).
Yu. V. Yushkova, E. I. Chernyak, Yu. F. Polienko, Yu. V. Gatilov, S. V. Morozov, and I. A. Grigor’ev, Chem. Nat. Compd., 49, 253 (2013).
H. Fonge, S. K. Chitneni, J. Lixin, K. Vunckx, K. Prinsen, J. Nuyts, L. Mortelmans, G. Bormans, Y. Ni, and A. Verbruggen, Bioconjugate Chem., 18, 1924 (2007).
M. Koufaki, A. Detsi, E. Theodorou, C. Kiziridi, T. Calogeropoulou, A. Vassilopoulos, A. P. Kourounakis, E. Rekka, P. N. Kourounakis, C. Gaitanaki, and P. Papazafiri, Bioorg. Med. Chem., 12, 4835 (2004).
G. Socrates, Infrared Characteristic Group Frequencies: Tables and Charts, 2nd Ed., Wiley, New York, 1994, p. 249.
M. Gorecki, A. Suszczynska, M. Woznica, A. Baj, M. Wolniak, M. K. Cyranski, S. Witkowski, and J. Frelek, Org. Biomol. Chem., 12, 2235 (2014).
O. D. Jankowski, K. E. Wesson, et al., US Pat. Appl. No. 20090118257 A1, May 7, 2009.
H. Gunther, NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry, 3rd Ed., 2013; ISBN: 978-3-527-33004-1, p. 734.
RF State Pharmacopoeia, XIIth Ed., Moscow, 2007, p. 92.
Y. Catel, F. Aladedunya, and R. Przybylski, J. Agric. Food Chem., 58, 11081 (2010).
Acknowledgment
Spectra of the synthesized compounds were obtained at the Khimiya CCU, SB, RAS. We thank Cand. Chem. Sci. A. M. Genaev for discussing the spectral data and Cand. Chem. Sci. N. I. Tkacheva for information support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2015, pp. 922–925.
Rights and permissions
About this article
Cite this article
Yushkova, Y.V., Chernyak, E.I., Morozov, S.V. et al. Antioxidants Based on Covalently and Ionically Bound Trolox Conjugates. Chem Nat Compd 51, 1070–1073 (2015). https://doi.org/10.1007/s10600-015-1494-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1494-2