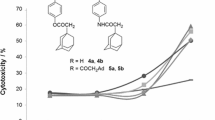
A series of novel 2-phenyl-3H-imidazo[4,5-b]pyridines and 2-phenyl-3H-imidazo[4,5-c]pyridines and their precursors were synthesized. Their in vitro cytotoxicity against MCF-7 human breast adenocarcinoma cell line has been investigated, and some of the tested compounds have shown high cytotoxic activity against MCF-7 cells. N-Hydroxy-4-(3H-imidazo[4,5-b]pyridin-2-yl)benzenecarboximidamide was the most active compound with IC50 equal to 0.082 μM, which is an activity almost as high as that of a commonly used anticancer drugs docetaxel and imatinib mesylate.









Similar content being viewed by others
References
Thurston, D. E. Chemistry and Pharmacology of Anticancer Drugs, 2nd ed.; CRC Press: Boca Raton, 2013.
Hranjec, M.; Lučić, B.; Ratkaj , I.; Pavelić, S. K.; Piantanida, I.; Pavelić, K.; Karminski-Zamola, G. Eur. J. Med. Chem. 2011, 46, 2748.
Zhang, M.; Yogesha, S. D.; Mayfield, J. E.; Gill, G. N.; Zhang, Y. Febs J. 2013, 280, 4739.
Lukasik, P. M.; Elabar, S.; Lam, F.; Shao, H.; Liu, X.; Abbas, A.Y.; Wang, S. Eur. J. Med. Chem. 2012, 57, 311.
Bavetsias, V.; Sun, C.; Bouloc, N.; Reynisson, J.; Workman, P.; Linardopoulos, S.; McDonald, E. Bioorg. Med. Chem. Lett. 2007, 17, 6567.
Bavetsias, V.; Large, J. M.; Sun, C.; Bouloc, N.; Kosmopoulou, M.; Matteucci, M.; Wilsher, N. E.; Martins, V.; Reynisson, J.; Atrash, B.; Faisal, A.; Urban, F.; Valenti, M.; de Haven Brandon, A.; Box, G.; Raynaud, F. I.; Workman, P.; Eccles, S. A.; Bayliss, R.; Blagg, J.; Linardopoulos, S.; McDonald, E. J. Med. Chem. 2010, 53, 5213.
Bavetsias, V.; Crumpler, S.; Sun, C.; Avery, S.; Atrash, B.; Faisal, A.; Moore, A. S.; Kosmopoulou, M.; Brown, N.; Sheldrake, P. W.; Bush, K.; Henley, A.; Box, G.; Valenti, M.; de Haven Brandon, A.; Raynaud, F. I.; Workman, P.; Eccles, S. A.; Bayliss, R.; Linardopoulos, S.; Blagg, J. J. Med. Chem. 2012, 55, 8721.
Bavetsias, V.; Faisal, A.; Crumpler, S.; Brown, N.;Kosmopoulou, M.; Joshi, A.; Atrash, B.; Pérez-Fuertes, Y.; Schmitt, J. A.; Boxall, K. J.; Burke, R.; Sun, C.; Avery, S.; Bush, K.; Henley, A.; Raynaud, F. I.; Workman, P.; Bayliss, R.; Linardopoulos, S.; Blagg, J. J. Med. Chem. 2013, 56, 9122.
Wang, T.; Block, M. A.; Cowen, S.; Davies, A. M.; Devereaux, E.; Gingipalli, L.; Johannes, J.; Larsen, N. A.; Su, Q.; Tucker, J. A.; Whitston, D.; Wu, J.; Zhang, H. J.; Zinda, M.; Chuaqui, C. Bioorg. Med. Chem. Lett. 2012, 22, 2063.
Johannes, J. W.; Chuaqui, C.; Cowen, S.; Devereaux, E.; Gingipalli, L.; Molina, A.; Wang, T.; Whitston, D.; Wu, X.; Zhang, H.-J.; Zinda, M. Bioorg. Med. Chem. Lett. 2014, 24, 1138.
Cho, Y. S.; Angove, H.; Brain, C.; Chen, C. H.-T.; Cheng, H.; Cheng, R.; Chopra, R.; Chung, K.; Congreve, M.; Dagostin, C.; Davis, D. J.; Feltell, R.; Giraldes, J.; Hiscock, S. D.; Kim, S.; Kovats, S.; Lagu, B.; Lewry, K.; Loo, A.; Lu, Y.; Luzzio, M.; Maniara, W.; McMenamin, R.; Mortenson, P. N.; Benning, R.; O'Reilly, M.; Rees, D. C.; Shen, J.; Smith, T.; Wang, Y.; Williams, G.; Woolford, A. J. A.; Wrona, W.; Xu, M.; Yang, F.; Howard, S. ACS Med. Chem. Lett. 2012, 3, 445.
Chen, D.; Wang, Y.; Ma, Y.; Xiong, B.; Ai, J.; Chen, Y.; Geng, M.; Shen, J. Chem Med Chem. 2012, 7, 1057.
Munde, M.; Kumar, A.; Peixoto, P.; Depauw, S.; Ismail, M. A.; Farahat, A. A.; Paul, A.; Say, M. V.; David-Cordonnier, M.-H.; Boykin, D. W.; Wilson, W. D. Biochemistry 2014, 53, 1218.
Paul, A.; Bhattacharya, S. Curr. Sci. 2012, 102, 212.
Baraldi, P.G.; Bovero, A.; Fruttarolo, F.; Preti, D.; Tabrizi, M. A.; Pavani, M. G.; Romagnoli, R. Med. Res. Rev. 2004, 24, 475.
Karataş, H.; Alp, M.; Yildiz, S.; Göker, H. Chem. Biol. Drug. Res. 2012, 80, 237.
Püsküllü, M. O.; Yildiz, S.; Duydu, Y.; Üstündag, A. J. Enzyme Inhib. Med. Chem. 2015, 30, 173.
Rice, C. A.; Colon, B. L.; Alp, M.; Göker H.; Boykin, D. W.; Kyle, D. E. Antimicrob. Agents Chemother. 2015, 59, 2037.
Ridley, H. F.; Spickett, R. G. W.; Timmis, G. M. J. Heterocycl. Chem. 1965, 2, 456.
Khanna, I. K.; Weier, R. M,; Lentz, K. T.; Swenton, L.; Lankin, D. C. J. Org. Chem. 1995, 60, 960
Zelenin, K. N.; Ukraintsev, I. V.; Alekseev, V. V. Chem. Heterocycl. Compd. 1998, 34, 329. [Khim. Geterotsikl. Soedin. 1998, 363.]
Suzuki, H.; Ohashi, M.; Itoh, K.; Matsuda, I.; Ishii, Y. Bull. Chem. Soc. Jpn. 1975, 48, 1922.
Yu, J.; Lu, M. Synth. Commun. 2014, 44, 2520.
Lipunova, G. N.; Nosova, E. V.; Charushin, V. N. Chem. Heterocycl. Compd. 2014, 50, 764. [Khim. Geterotsikl. Soedin. 2014, 831.]
Roger, R. Neilson, D. Chem. Rev. 1961, 61, 179.
Mosmann, T. J. Immunol. Methods 1983, 65, 55.
Kuźma, Ł.; Wysokińska, H.; Różalski, M.; Krajewska, U.; Kisiel, W. Fitoterapia 2012, 83, 770.
Malamas, M. S.; Sredy, J.; Moxham, C.; Katz, A.; Xu, W.; McDevitt, R.; Adebayo, F. O.; Sawicki, D. R.; Seestaller, L.; Sullivan, D.; Taylor, J. R. J. Med. Chem. 2000, 43, 1293.
The Central Laboratory of Ankara University, Faculty of Pharmacy, provided support for the acquisition of the NMR and mass spectra and elemental analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(8), 723–733
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information
(PDF 4352 kb)
Rights and permissions
About this article
Cite this article
Püsküllü, M.O., Karaaslan, C., Bakar, F. et al. Synthesis and potent cytotoxicity of some novel imidazopyridine derivatives against MCF-7 human breast adenocarcinoma cell line. Chem Heterocycl Comp 51, 723–733 (2015). https://doi.org/10.1007/s10593-015-1765-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1765-7