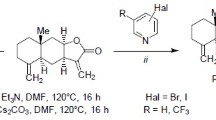
An aza-Michael reaction of isoalantolactone with 5-bromo- or 5-iodo-substituted uracils gave (11R)-13-[5-bromo(iodo)-2,4-dioxotetrahydropyrimidin-1-yl]eudesma-4(15)-en-8β,12-olides, which were highly active in Pd-catalyzed cross-coupling reaction with terminal alkynes. Copper-catalyzed Mannich reaction of (11R)-13-(5-ethynyl-2,4-dioxotetrahydropyrimidin-1-yl)eudesma-4(15)-en-8β,12-olide with secondary amines and formaldehyde was used for the synthesis of (11R)-13-[5-(diethyl-amino)propynyl]-, (11R)-13-[5-(pyrrolidinyl-1-yl)propynyl]-, (11R)-13-[5-(4-oxopiperidin-1-yl)-propynyl]-, (11R)-13-[5-(4-methylpiperazin-1-yl)propynyl]-, and (11R)-13-(5-{[2-(pyridin-3-yl)piperidin-1-yl]propynyl}-2,4-dioxotetrahydropyrimidin-1-yl)eudesmanolides. The treatment of 13-[5(propargyl-amino)tetrahydropyrimidin-1-yl]eudesmanolides with silver nitrate led to the corresponding (11R)-13-(2-oxofuro[2,3-d]pyrimidin-3(2H)-yl)eudesmanolides. The structures of two compounds were proved by X-ray structural analysis.

Similar content being viewed by others
References
S. S. Patrushev, M. M. Shakirov, T. V. Rybalova, and E. E. Shul’ts, Zh. Org. Khim., 49, 1802 (2013). [Russ. J. Org. Chem., 49, 1783 (2013).]
A. Gangjee, Y. Zeng, J. J. McGuire, and R. L. Kisliuk, J. Med. Chem., 48, 5329 (2005).
F. Amblard, V. Aucagne, P. Guenot, R. F. Schinazi, and L. A. Agrofoglio, Bioorg. Med. Chem., 13, 1239 (2005).
T. Gazivoda, M. Šokčević, M. Kralj, L. Šuman, K. Pavelić, E. De Clercq, G. Andrei, R. Snoeck, J. Balzarini, M. Mintas, and S. Raić-Malić, J. Med. Chem., 50, 4105 (2007).
M. J. Robins, I. Nowak, V. K. Rajwanshi, K. Miranda, J. F. Cannon, M. A. Peterson, G. Andrei, R. Snoeck, E. De Clercq, and J. Balzarini, J. Med. Chem., 50, 3897 (2007).
N. Foloppe, L. M. Fisher, R. Howes, P. Kierstan, A. Potter, A. G. S. Robertson, and A. E. Surgenor, J. Med. Chem., 48, 4332 (2005).
Y. Miyazaki, Y. Maeda, H. Sato, M. Nakano, and G. W. Mellor, Bioorg. Med. Chem. Lett., 18, 1967 (2008).
A. Zhao, X. Gao, Y. Wang, J. Ai, Y. Wang, Y. Chen, M. Geng, and A. Shang, Bioorg. Med. Chem., 19, 3906 (2011).
X. Y. Jiao, D. J. Kopecky, J. S. Liu, J. Q. Liu, J. C. Jaen, M.G. Cardozo, R. Sharma, N. Walker, H. Wesche, S. Li, E. Farrelly, S.-H. Xiao, Z. Wang, and F. Kayser, Bioorg. Med. Chem. Lett., 22, 6212 (2012).
P. A. Harris, D. Bandyopadhyay, S. B. Berger, N. Campobasso, C. A. Capriotti, J. A. Cox, L. Dare, J. N. Finger, S. J. Hoffman, K. M. Kahler, R. Lehr, J. D. Lich, R. Nagilla, R. T. Nolte, M. T. Ouellette, C. S. Pao, M. C. Schaeffer, A. Smallwood, H. H. Sun, B. A. Swift, R. D. Totoritis, P. Ward, R. W. Marquis, J. Bertin, and P. J. Gough, ACS Med. Chem. Lett., 4, 1238 (2013).
A. V. Belovodskii, E. E. Shults, M. M. Shakirov, I. Yu. Bagryanskaya, Yu. V. Gatilov, and G. A. Tolstikov, Zh. Org. Khim., 46, 1710 (2010). [Russ. J. Org. Chem., 46, 1719 (2010).]
E. E. Shults, A. V. Belovodskii, T. G. Tolstikova, M. P. Dolgikh, E. A. Morozova, and G. A. Tolstikov, RU Pat. 2413724; Byul. Izobret., No. 7, 10 (2011).
E. E. Shul’ts, A. V. Belovodskii, M. M. Shakirov, Yu. V. Gatilov, A. G. Pokrovskii, M. A. Pokrovskii, and G. A. Tolstikov, Khim. Prirodn. Soedin., 48, 215 (2012). [Chem. Nat. Compd., 48, 238 (2012).]
S. G. Klochkov, I. V. Anan’ev, S. A. Pukhov, and S. V. Afanas’eva, Khim. Geterotsikl. Soedin., 750 (2012). [Chem. Heterocycl. Compd., 48, 698 (2012).]
F. J. B. Mendonca, Jr., J. V. dos Anjos, D. Sinou, S. J. de Melo, and R. M. Srivastava, Synthesis, 1890 (2007).
N. Fresneau, M.-A. Hiebel, L. A. Agrofoglio, and S. Berteina-Raboin, Tetrahedron Lett., 53, 1760 (2012).
T. G. Kraljević, A. Bistrović, M. Dedić, S. K. Pavelić, M. Sedić, and S. Raić-Malić, Tetrahedron Lett., 53, 5144 (2012).
V. Aucagne, F. Amblard, and L. A. Agrofoglio, Synlett, 2406 (2004).
A. Sniady, A. Durham, M. S. Morreale, A. Marcinek, S. Szafert, T. Lis, K. R. Brzezinska, T. Iwasaki, T. Ohshima, K. Mashima, and R. Dembinski, J. Org. Chem., 73, 5881 (2008).
Z. Janeba, J. Balzarini, G. Andrei, R. Snoeck, E. De Clercq, and M. J. Robins, J. Med. Chem., 48, 4690 (2005).
S. A. Osadchii, E. E. Shults, E. V. Polukhina, M. M. Shakirov, S. F. Vasilevskii, A. A. Stepanov, and G. A. Tolstikov, Izv. Akad. Nauk, Ser. Khim., 1215 (2007). [Russ. Chem. Bull., Int. Ed., 56, 1261 (2007).]
V. T. Bauman, E. E. Shults, M. M. Shakirov, and G. A. Tolstikov, Zh. Org. Khim., 48, 1489 (2012). [Russ. J. Org. Chem., 48, 1473 (2012).]
R. Csuk, C. Nitsche, R. Sczepek, S. Schwarz, and B. Siewert, Arch. Pharm. Chem. Life Sci., 346, 232 (2013).
K. Nakanishi, Infrared Spectra and the Structure of Organic Compounds [Russian translation], Mir, Moscow (1965), p. 33.
F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., B58, 380 (2002).
M. Currie and G. A. Sim, J. Chem. Soc., Perkin Trans. 2, 400 (1973).
R. S. Rowland and R. Taylor, J. Phys. Chem., 100, 7384 (1996).
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor, J. Chem. Soc., Perkin Trans. 2, S1 (1987).
U. M. Dzhemilev, N. R. Popod’ko, and E. V. Kozlova, Metal Complex Catalysis in Organic Synthesis [in Russian], Khimiya, Moscow (1999), p.104.
S. C. Srivastava, M. M. Mehra, G. K. Trivedi, and S. C. Bhattacharyya, Indian J. Chem., 9, 512 (1971).
A. S. Sadykov, Chemistry of Anabasis aphylla alkaloids [in Russian], Izd-vo Academy of Sciences of Uzbekistan SSR, Tashkent (1956), p. 166.
G. M. Sheldrick, SADABS. Version 2.01, Bruker AXS Inc. Madison, Wisconsin, USA (2004).
G. M. Sheldrick, SHELXS-97 – Programs for Crystal Structure Analysis (Release 97-2), Univ. Göttingen, Germany (1997).
G. M. Sheldrick, SHELXL-97 – A Program for Exploiting the Redundancy of Area-detector X-ray Data, Univ. Göttingen, Germany (1999).
This work was performed with financial support from the Russian Science Foundation (project 14-13-00822) and the Grants Council of the President of the Russian Federation (grant NSh-2625.2014.3).
The analytical and spectral studies were performed at the Collective Use Center of the Chemical Services Center, Siberian Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
*For Communication 7, see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 8, pp. 1155-1173, August, 2014.
Rights and permissions
About this article
Cite this article
Patrushev, S.S., Shakirov, M.M., Rybalova, T.V. et al. Synthetic Transformations of Sesquiterpene Lactones. 8*. Synthesis of 13-(2-Oxofuro- [2,3-d]pyrimidin-3(2H)-yl)eudesmanolides. Chem Heterocycl Comp 50, 1063–1080 (2014). https://doi.org/10.1007/s10593-014-1566-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1566-4