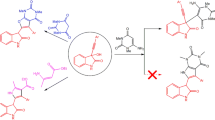
A simple method is proposed for the synthesis of 1,2,3-thiadiazolylureas by the reaction of 1,2,3-thia-diazolyl isocyanates with primary amines. 1,2,3-Thiadiazolyl isocyanates were obtained in situ by the Curtius rearrangement of 1,2,3-thiadiazolylcarbonyl azides. Cytokinin activity was tested for N-(4-methyl-1,2,3-thiadiazol-5-yl)-N'-(4-methylphenyl)urea, which is an analog of thidiazuron, differing in the presence of two methyl groups in the molecule.



Similar content being viewed by others
References
M. L. Petrov, M. Iekhlev, and F. S. Teplyakov, Zh. Org. Chem., 48, 728 (2012). [Russ. J. Org. Chem., 48, 728 (2012).
N. P. Bel’skaya, A. I. Bolgova, M. L. Kondrat’eva, O. S. El’tsov, and B. A. Bakulev, Izv. Akad. Nauk, Ser. Khim., 60, 876 (2011). [Russ. Chem. Bull., 60, 896 (2011)].
P. E. Prokhorova, T. V. Glukhareva, L. V. Dyudya, E. A. Alekseeva, and Yu. Yu. Morzherin, Izv. Akad. Nauk, Ser. Khim., 59, 848 (2010). [Russ. Chem. Bull., 59, 867 (2010)].
Yu. Yu. Morzherin, P. E. Prokhorova, D. A. Musikhin, T. V. Glukhareva, and Zh. H. Fan, Pure Appl. Chem., 83, 715 (2011).
D.-D. Guo, D. Wang, Z.-J. Fan, J.-J. Li, H.-B. Song, Q. Fan, T. A. Kalinina, Y. Y. Morzherin, N. P. Belskaya, and V. A. Bakulev, Chin. J. Chem., 31, 1721 (2012).
W. T. Mao, D. D. Guo, Z. J. Fan, X.-Sh. Gu, H. B. Song, D. Wang, Q. Fan, T. A. Kalinina, Y. Y. Morzherin, and V. A. Bakulev, Chin. J. Struct. Chem., 32, 357 (2013).
J. Ryals, S. Uknes, and E. Ward, Plant Physiol., 104, 1109 (1994).
K. A. Lawton, L. Friedrich, M. Hunt, K. Weymann, T. Delaney, H. Kessmann, Th. Staub, and J. Ryals, Plant J., 10, 71 (1996).
Z. Azami-Sardooei, H. S. Seifi, D. Vleesschauwer, and M. Höfte, Australas. Plant Pathol., 42, 485 (2013).
K. Tsubata, O. Sanpei, K. Takagi, K. Umetani, T. Uchikurohane, and S. Tajima, WO Pat. Appl. 9923084.
M. Yasuda, M. Kusajima, M. Nakajima, K. Akutsu, T. Kudo, Sh. Yoshida, and H. Nakashita, J. Pestic. Sci., 31, 329 (2006).
H. Schulz and F. Arndt, DE Pat. Appl. 2214632.
A. R. Gill and P. Siwach, Res. J. BioTechnol., 9, 63 (2014).
C. A. Huetteman and J. E. Preece, Plant Cell, Tissue Organ Cult., 33, 105 (1993).
R. U. Rehman, M. F. Chaudhary, K. M. Khawar, G. Lu, A. Mannan, and M. Zia, Biologia, 69, 341 (2014).
P. Muthuppalaniappan, S. Viswanadha, G. S. Merikapudi, and S. K. Vakkalanka, WO Pat. Appl. 2011042797.
A. Abad, C. Agulló, A. C. Cuñat, R. Jiménez, and C. Vilanova, J. Agric. Food Chem., 52, 4675 (2004).
R. Raap and R. G. Micetich, Can. J. Chem., 46, 1057 (1968).
W.-M. Xu, Sh.-Z. Li, M. He, S. Yang, P. Li, and X.-Y. Li, Bioorg. Med. Chem. Lett., 23, 5821 (2013).
J. Wolff, Liebigs Ann. Chem., 333, 6 (1904).
R. A. Fletcher and D. McCullagh, Planta, 101, 88 (1971).
R. A. Fletcher, V. Kallidumbil, and S. N. Bhardwaj, Plant Cell Physiol., 23, 717 (1982).
A. J. Al Mansouri and S. S. Kurup, Emirates J. Food Agric., 21, 48 (2009).
J. Guo, X. Hu, and R. Duan, J. Plant Growth Regul., 24, 93 (2005).
B. Rasulov, I. Bichele, A. Laisk, and Ü. Niinemets, Plant Cell Environ., 37, 724 (2014).
E. A. Burkhanova, A. B. Fedina, Yu. A. Baskakov, and O. N. Kulaeva, Fiziologiya Rastenii, 31, 13 (1984). [Russ. J. Plant Physiol., 31, 13 (1984)].
N. M. Zhirmunskaya, T. V. Ovsyannikova, A. A. Shapovalov, and Yu. A. Baskakov, Fiziologiya i Biokhimiya Kul'turnykh Rastenii, 21, 446 (1989).
Yu. A. Baskakov, A. A. Shapovalov, N. M. Zhirmunskaya, and T. V. Ovsyannikova, Dokl. Akad. Nauk SSSR, 257, 1514 (1981).
N. L. Klyachko, I. M. Shramm, and O. N. Kulaeva, Fiziologiya Rastenii, 34, 319 (1987). [Russ. J. Plant Physiol., 34, 319 (1987)].
V. A. Klimova, Basic Micromethods for the Analysis of Organic Compounds [in Russian], Khimiya, Moscow (1967), p. 101.
A. R. Jalilian, S. Sattari, M. Bineshmarvasti, A. Shafiee, and M. Daneshtalab, Arch. Pharm., 333, 347 (2000).
D. L. Pain and R. Slack, J. Chem. Soc., 5166 (1965).
This work was carried out with the financial support of the Russian Foundation for Basic Research (grant 13-03-00137 A).
Author information
Authors and Affiliations
Corresponding author
Additional information
*Dedicated to Academician O. N. Chupakhin on the occasion of his 80th birthday.
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 1127-1134, July, 2014.
Rights and permissions
About this article
Cite this article
Kalinina, T.A., Shakhmina, Y.S., Glukhareva, T.V. et al. 1,2,3-Thiadiazolyl Isocyanates in the Synthesis of Biologically Active Compounds. Study of the Cytotoxic Activity of N-(4-methyl-1,2,3-thiadi-azolyl-5-yl)-N'-(4-methylphenyl)Urea*. Chem Heterocycl Comp 50, 1039–1046 (2014). https://doi.org/10.1007/s10593-014-1561-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1561-9