Abstract
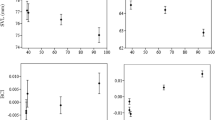
Molecular markers can reveal interesting aspects of organismal ecology and evolution, especially when surveyed in rare or elusive species. Herein, we provide a preliminary assessment of golden eagle (Aquila chrysaetos) population structure in North America using novel single nucleotide polymorphisms (SNPs). These SNPs included one molecular sexing marker, two mitochondrial markers, 85 putatively neutral markers that were derived from noncoding regions within large intergenic intervals, and 74 putatively nonneutral markers found in or very near protein-coding genes. We genotyped 523 eagle samples at these 162 SNPs and quantified genotyping error rates and variability at each marker. Our samples corresponded to 344 individual golden eagles as assessed by unique multilocus genotypes. Observed heterozygosity of known adults was significantly higher than of chicks, as was the number of heterozygous loci, indicating that mean zygosity measured across all 159 autosomal markers was an indicator of fitness as it is associated with eagle survival to adulthood. Finally, we used chick samples of known provenance to test for population differentiation across portions of North America and found pronounced structure among geographic sampling sites. These data indicate that cryptic genetic population structure is likely widespread in the golden eagle gene pool, and that extensive field sampling and genotyping will be required to more clearly delineate management units within North America and elsewhere.





Similar content being viewed by others
References
Anderson EC, Dunham KK (2008) The influence of family groups on inferences made with the program Structure. Mol Ecol Resour 8:1219–1229. doi:10.1111/j.1755-0998.2008.02355.x
Avise JC (1994) Molecular markers, natural history and evolution. Chapman and Hall, New York
Avise JC (2000) Phylogeography: The history and formation of species. Harvard University Press, Cambridge
Beerli P (2004) Effect of unsampled populations on the estimation of population sizes and migration rates between sampled populations. Mol Ecol 13:827–836. doi:10.1111/j.1365-294X.2004.02101.x
Bekkevold D, Helyar SJ, Limborg MT et al (2015) Gene-associated markers can assign origin in a weakly structured fish, Atlantic herring. ICES J Mar Sci 72:1790–1801
Bloom P, Clark W (2001) Molt and sequence of plumages of golden eagles and a technique for in-hand ageing. North Am Bird Bander 26:97–116
Bourke BP, Frantz AC, Lavers CP et al (2010) Genetic signatures of population change in the British golden eagle (Aquila chrysaetos). Conserv Genet 11:1837–1846. doi:10.1007/s10592-010-0076-x
Cadahía L, Pinsker W, Josénegro J et al (2009) Repeated sequence homogenization between the control and pseudo-control regions in the mitochondrial genomes of the subfamily aquilinae. J Exp Zool Part B 312:171–185. doi:10.1002/jez.b.21282
Cai Q, Qian X, Lang Y, Luo Y, Xu J, Pan S, Hui Y, Gou C, Cai Y, Hao M, Zhao J, Wang S, Wang Z, Zhang X, He R, Liu J, Luo L, Li Y, Wang J (2013) Genome sequence of ground tit Pseudopodoces humilis and its adaptation to high altitude. Genome Biol 14:R29
Chakraborty R (1981) The distribution of the number of heterozygous loci in an individual in natural populations. Genetics 98:461–466
Chapman JR, Nakagawa S, Coltman DW et al (2009) A quantitative review of heterozygosity-fitness correlations in animal populations. Mol Ecol 18:2746–2765. doi:10.1111/j.1365-294X.2009.04247.x
Clegg MT, Allard RW (1973) Viability versus fecundity selection in the slender wild oat, Avena barbata L. Science 181:667–668
Cohas A, Bonenfant C, Kempenaers B, Allainé D (2009) Age-specific effect of heterozygosity on survival in alpine marmots, Marmota marmota. Mol Ecol 18:1491–1503. doi:10.1111/j.1365-294X.2009.04116.x
DePristo MA, Banks E, Poplin R et al (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43:491–498. doi:10.1038/ng.806
DeWoody YD, DeWoody JA (2005) On the estimation of genome-wide heterozygosity using molecular markers. J Hered 96:85–88. doi:10.1093/jhered/esi017
Do C, Waples RS, Peel D et al (2014) NeEstimator V2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Doran AG, Creevey CJ (2013) Snpdat: easy and rapid annotation of results from de novo snp discovery projects for model and non-model organisms. BMC Bioinformatics 14:45. doi:10.1186/1471-2105-14-45
Downing T, Lloyd AT, O’Farrelly C, Bradley DG (2010) The differential evolutionary dynamics of avian cytokine and TLR gene classes. J Immunol 184:6993–7000. doi:10.4049/jimmunol.0903092
Doyle JM, Katzner TE, Bloom PH et al (2014) The genome sequence of a widespread apex predator, the golden eagle (Aquila chrysaetos). PLoS ONE 9:20–22. doi:10.1371/journal.pone.0095599
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. doi:10.1007/s12686-011-9548-7
Edwards SV, Gasper J, March M (1998) Genomics and polymorphism of Agph-DAB1, an Mhc class II B gene in red-winged blackbirds (Agelaius phoeniceus). Mol Biol Evol 15:236–250
Ekblom R, French L, Slate J, Burke T (2010) Evolutionary analysis and expression profiling of zebra finch immune genes. Genome Biol Evol 2:781–790. doi:10.1093/gbe/evq061
Evans S, Sheldon B (2008) Interspecific patterns of genetic diversity in birds: correlations with extinction risk. Conserv Biol 22:1016–1025
Evans PD, Vallender EJ, Lahn BT (2006) Molecular evolution of the brain size regulator genes CDK5RAP2 and CENPJ. Gene 375:75–79. doi:10.1016/j.gene.2006.02.019
Ferchaud A-L, Pedersen SH, Bekkevold D et al (2014) A low-density SNP array for analyzing differential selection in freshwater and marine populations of threespine stickleback (Gasterosteus aculeatus). BMC Genom 15:867. doi:10.1186/1471-2164-15-867
Frankham R (1995) Effective population size/adult population size ratios in wildlife: a review. Genet Res 66:95–107. doi:10.1017/S0016672308009695
Fraser DJ, Bernatchez L (2001) Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Mol Ecol 10:2741–2752
Fridolfsson A-K, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Funk WC, McKay JK, Hohenlohe PA, Allendorf FW (2012) Harnessing genomics for delineating conservation units. Trends Ecol Evol 27:489–496
Galpern P, Manseau M, Hettinga P et al (2012) Allelematch: an R package for identifying unique multilocus genotypes where genotyping error and missing data may be present. Mol Ecol Resour 12:771–778. doi:10.1111/j.1755-0998.2012.03137.x
Garner A, Rachlow J, Hicks J (2005) Patterns of genetic diversity and its loss in mammalian populations. Conserv Biol 19:1215–1221
Grueber CE, Walls CP, Jamieson IG (2013) Genetic drift outweighs natural selection at toll-like receptor (TLR) immunity loci in a re-introduced population of a threatened species. Mol Ecol 22:4470–4482
Grueber CE, Knafler GJ, King TM, Senior AM, Grosser S, Robertson B, Weston KA, Brekke P, Harris CL, Jamieson IG (2015) Toll-like receptor diversity in 10 threatened bird species: relationship with microsatellite heterozygosity. Conserv Genet 16:595–611
Haasl R, Payseur BA (2016) Fifteen years of genomewide scans for selection: trends, lessons and unaddressed genetic sources of complication. Mol Ecol 25:5–23
Hailer F, Helander B, Folkestad AO et al (2007) Phylogeography of the white-tailed eagle, a generalist with large dispersal capacity. J Biogeogr 34:1193–1206. doi:10.1111/j.1365-2699.2007.01697.x
Hoffman JI, Simpson F, David P et al (2014) High-throughput sequencing reveals inbreeding depression in a natural population. Proc Natl Acad Sci USA 111:3775–3780. doi:10.1073/pnas.1318945111
Hull JM, Hull AC, Sacks BN et al (2008) Landscape characteristics influence morphological and genetic differentiation in a widespread raptor (Buteo jamaicensis). Mol Ecol 17:810–824. doi:10.1111/j.1365-294X.2007.03632.x
Jollie M (1947) Plumage changes in the Golden Eagle. Auk 64:549–576
Katzner T, Smith BW, Miller TA et al (2012) Status, biology and conservation priorities for North America’s Eastern Golden Eagle (Aquila chrysaetos) population. Auk 129:168–176
Katzner TE, Nelson DM, Braham MA, et al (forthcoming) Golden eagle fatalities demonstrate the continental-scale consequences of local-scale renewable energy conservation
Keenan K, McGinnity P, Cross T et al (2013) DiveRsity: an R package for the estimation of population genetics parameters and their associated errors. Methods Ecol Evol 4:782–788
Keller L, Waller D (2002) Inbreeding effects in wild populations. Trends Ecol Evol 16:1099–1106
Kosiol C, Vinař T, da Fonseca RR et al (2008) Patterns of positive selection in six mammalian genomes. PLoS Genet 4(8):e1000144
Lampila S, Orell M, Kvist L (2011) Willow tit Parus montanus extrapair offspring are more heterozygous than their maternal half-siblings. J Avian Biol 42:355–362. doi:10.1111/j.1600-048X.2011.05349.x
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi:10.1093/bioinformatics/btp324
Limborg MT, Helyar SJ, De Bruyn M et al (2012) Environmental selection on transcriptome-derived SNPs in a high gene flow marine fish, the Atlantic herring (Clupea harengus). Mol Ecol 21:2355–2356
Malenfant RM, Coltman DW, Davis CS (2015) Design of a 9 K illumina BeadChip for polar bears (Ursus maritimus) from RAD and transcriptome sequencing. Mol Ecol Resour 15:587–600. doi:10.1111/1755-0998.12327
McIntyre CL, Collopy MW, Lindberg MS (2006) Survival probability and mortality of migratory juvenile golden eagles from interior Alaska. J Wildl Manage 70:717–722
McIntyre CL, Douglas DC, Collopy MW (2008) Movements of golden eagles (Aquila chrysaetos) from interior Alaska during their first year of independence. Auk 125:214–224. doi:10.1525/auk.2008.125.1.214
Millsap G, Zimmerman G, Sauer J et al (2013) Golden eagle population trends in the western United States: 1968-2010. J Wildl Manage 77:1436–1448
Millsap B, Harmata A, Stahlecker D, Mikesic D (2014) Natal dispersal distance of Bald and Golden eagles originating in the Coterminous United States as inferred from band encounters. J Raptor Res 48:2014
Mitton JB (1997) Selection in natural populations. Oxford University Press, New York
Mitton JB, Pierce BA (1980) The distribution of individual heterozygosity in natural populations. Genetics 95:1043–1054
Moritz C (1994) Defining ‘Evolutionary Significant Units’ for conservation. Trends Ecol Evol 9:373–375
Morneau F, Tremblay JA, Todd C et al (2015) Known breeding distribution and abundance of golden eagles in Eastern North America. Northeastern Naturalist 22:236–247
Mueller JC, Korsten P, Hermannstaedter C et al (2013) Haplotype structure, adaptive history and associations with exploratory behaviour of the DRD4 gene region in four great tit (Parus major) populations. Mol Ecol 22:2797–2809. doi:10.1111/mec.12282
Nam K, Mugal C, Nabholz B et al (2010) Molecular evolution of genes in avian genomes. Genome Biol 11:R68. doi:10.1186/gb-2010-11-6-r68
Nebel C, Gamauf A, Haring E et al (2015) Mitochondrial DNA analysis reveals Holarctic homogeneity and a distinct Mediterranean lineage in the Golden eagle (Aquila chrysaetos). Biol J Linn Soc 116:328–340
Neph S, Kuehn MS, Reynolds AP et al (2012) BEDOPS: high-performance genomic feature operations. Bioinformatics 28:1919–1920. doi:10.1093/bioinformatics/bts277
Nery MF, Gonzalez DJ, Opazo JC (2013) How to make a dolphin: molecular signature of positive selection in Cetacean Genome. PLoS One 8(6):e65491
Ogden R, Heap E, Mcewing R et al (2015) Population structure and dispersal patterns in Scottish Golden Eagles Aquila chrysaetos revealed by molecular genetic analysis of territorial birds. Ibis (Lond 1859). doi:10.1111/ibi.12282
Ovenden JR, Morgan JAT, Street R et al (2011) Negligible evidence for regional genetic population structure for two shark species Rhizoprionodon acutus (Ruppell, 1837) and Sphyrna lewini (Griffith & Smith, 1834) with contrasting biology. Mar Biol 7:1497–1509
Palsbøll PJ, Bérubé M, Allendorf FW (2007) Identification of management units using population genetic data. Trends Ecol Evol 22:11–16. doi:10.1016/j.tree.2006.09.003
Palstra F, Ruzzante D (2008) Genetic estimates of contemporary effective population size: what can they tell us about the importance of genetic stochasticity for wild population persistence? Mol Ecol 17:3428–3447. doi:10.1111/j.1365-294X.2008.03842.x
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research–an update. Bioinformatics 28:2537–2539. doi:10.1093/bioinformatics/bts460
Pritchard JK (2010) Documentation for structure software: Version 2.3. http://pritchardlab.stanford.edu/structure_software/release_versions/v2.3.4/structure_doc.pdf. Accessed 27 May 2015
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qu Y, Zhao H, Han N et al (2013) Ground tit genome reveals avian adaptation to living at high altitudes in the Tibetan plateau. Nat Commun 4:2071. doi:10.1038/ncomms3071
Rands CM, Darling A, Fujita M et al (2013) Insights into the evolution of Darwin’s finches from comparative analysis of the Geospiza magnirostris genome sequence. BMC Genom 14:95. doi:10.1186/1471-2164-14-95
Robinson JT, Thorvaldsdóttir H, Winckler W et al (2011) Integrative genomics viewer. Nat Biotechnol 29:24–26
Rubin C-J, Zody MC, Eriksson J et al (2010) Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464:587–591. doi:10.1038/nature08832
Rudnick JA, Katzner TE, Bragin EA et al (2005) Using naturally shed feathers for individual identification, genetic parentage analyses, and population monitoring in an endangered Eastern imperial eagle (Aquila heliaca) population from Kazakhstan. Mol Ecol 14:2959–2967. doi:10.1111/j.1365-294X.2005.02641.x
Ruegg KC, Anderson EC, Paxton KL et al (2014) Mapping migration in a songbird using high-resolution genetic markers. Mol Ecol 23:5726–5739. doi:10.1111/mec.12977
Ryder OA (1986) Species conservation and systematics: the dilemma of subspecies. Trends Ecol Evol 1:9–10
Schunter C, Garza JC, Macpherson E, Pascual M (2014) SNP development from RNA-seq data in a nonmodel fish: how many individuals are needed for accurate allele frequency prediction? Mol Ecol Resour 14:157–165. doi:10.1111/1755-0998.12155
Senn H, Ogden R, Cezard T et al (2013) Reference-free SNP discovery for the Eurasian beaver from restriction site-associated DNA paired-end data. Mol Ecol 22:3141–3150. doi:10.1111/mec.12242
Shafer ABA, Wolf JBW, Alves PC et al (2015) Genomics and the challenging translation into conservation practice. Trends Ecol Evol 30:78–87
Shaffer HB, Minx P, Warren DE et al (2013) The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol 14:R28. doi:10.1186/gb-2013-14-3-r28
Sonsthagen SA, Coonan TJ, Latta BC et al (2012) Genetic diversity of a newly established population of golden eagles on the channel islands, California. Biol Conserv 146:116–122. doi:10.1016/j.biocon.2011.11.031
Spielman D, Brook B, Frankham R (2004) Most species are not driven to extinction before genetic factors impact them. Proc Natl Acad Sci 101:15261–15264
Swanson W, Yang Z, Wolfner M, Aquadro C (2001) Positive Darwinian selection in the evolution of mammalian female reproductive proteins. Proc Natl Acad Sci USA 98:2509–2514
Szulkin M, Bierne N, David P (2010) Heterozygosity-fitness correlations: a time for reappraisal. Evolution 64:1202–1217. doi:10.1111/j.1558-5646.2010.00966.x
Torgerson D, Kulanthinal R, Singh R (2002) Mammalian sperm proteins are rapidly evolving: evidence of positive selection in functionally diverse genes. Mol Biol Evol 19:1973–1980
Turelli M, Ginzburg LR (1983) Should individual fitness increase with heterozygosity? Genetics 104:191–209
Van der Auwera GA, Carneiro MO, Hartl C et al (2013) From fastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinform 43:11.10.1–11.10.33
Voight BF, Kudaravalli S, Wen X et al (2006) A map of recent positive selection in the human genome. PLoS Biol 4(3):e72
Wan Q-H, Pan S-K, Hu L et al (2013) Genome analysis and signature discovery for diving and sensory properties of the endangered Chinese alligator. Cell Res 23:1091–1105. doi:10.1038/cr.2013.104
Warren WC, Clayton DF, Ellegren H et al (2010) The genome of a songbird. Nature 464:757–762. doi:10.1038/nature08819
Watson J (2010) The golden eagle. Yale University Press, New Haven
Wheeler M (2014) The genetics of conservation translocations: a comparison of North American Golden eagles (Aquila chrysaetos canadensis) and Bald eagles (Haliaeetus leucocephalus),pp 1–220
Zhan X, Pan S, Wang J et al (2013) Peregrine and saker falcon genome sequences provide insights into evolution of a predatory lifestyle. Nat Genet 45:536–566. doi:10.1038/ng.2588
Acknowledgments
The authors thank A. Capparella, J. Cooper, D. Driscoll, J. Fallon, D. Kramar, M. Kuishn, M. Lanzone, T. Miller, R. Murphy, K. O’Malley, J. Papp, K. Rogers, S. Slater, D. Stafford, D. Stahlecker, S. Thomas, L. Tran, S. Van Arsdae, and D. Wilst for their assistance collecting golden eagle samples. Special thanks to J. Willoughby for assistance generating Fig. 1, M. Sundaram for assistance with statistics, and to DeWoody lab members for comments on earlier drafts of the manuscript. The Nature Conservancy provided permission to use their lands. This work was supported by the U.S. Fish and Wildlife Service, the U.S. Bureau of Land Management (award numbers L12AC20102, L11PX02237, and L12AC2010), the California Department of Fish and Wildlife (Agreement #P1182024), and the Provost’s Office at Purdue University (University Faculty Scholar program). Eagle tissue and feather samples were collected under appropriate scientific collecting permits. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doyle, J.M., Katzner, T.E., Roemer, G.W. et al. Genetic structure and viability selection in the golden eagle (Aquila chrysaetos), a vagile raptor with a Holarctic distribution. Conserv Genet 17, 1307–1322 (2016). https://doi.org/10.1007/s10592-016-0863-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0863-0