Abstract
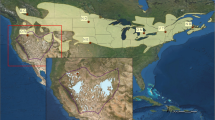
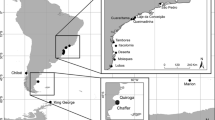
Migratory birds occupy geographically and ecologically disparate areas during their annual cycle with conditions on breeding and non-breeding grounds playing separate and important roles in population dynamics. We used data from nuclear microsatellite and mitochondrial DNA control region loci to assess the breeding and non-breeding spatial genetic structure of a transoceanic migrant shorebird, the bristle-thighed curlew. We found spatial variance in the distribution of allelic and haplotypic frequencies between the curlew’s two breeding areas in Alaska but did not observe this spatial structure throughout its non-breeding range on low-lying tropical and subtropical islands in the Central Pacific (Oceania). This suggests that the two breeding populations do not spatially segregate during the non-breeding season. Lack of migratory connectivity is likely attributable to the species’ behavior, as bristle-thighed curlews exhibit differential timing of migration and some individuals move among islands during non-breeding months. Given the detrimental impact of many past and current human activities on island ecosystems, admixture of breeding populations in Oceania may render the bristle-thighed curlew less vulnerable to perturbations there, as neither breeding population will be disproportionally affected by local habitat losses or by stochastic events. Furthermore, lack of migratory connectivity may enable bristle-thighed curlews to respond to changing island ecosystems by altering their non-breeding distribution. However, availability of suitable non-breeding habitat for curlews in Oceania is increasingly limited on both low-lying and high islands by habitat loss, sea level rise, and invasive mammalian predators that pose a threat to flightless and flight-compromised curlews during the molting period.



Similar content being viewed by others
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Avise JC (2004) Molecular markers, natural history and evolution, 2nd edn. Sinauer Associates Inc., Sunderland
Bandelt HJ, Forster P, Sykes BC, Richards MB (1995) Mitochondrial portraits of human populations. Genetics 141:743–753
Beerli P, Felsenstein J (1999) Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics 152:763–773
Beerli P, Felsenstein J (2001) Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc Natl Acad Sci USA 98:4563–4568
BirdLife International (2013) Species factsheet: Numenius tahitiensis. http://www.birdlife.org. Accessed 17 May 2013
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on Oceanic islands. Science 305:1955–1958
Boulet M, Norris DR (2006) The past and present of migratory connectivity. In: Boulet M, Norris DR (eds.) Patterns of migratory connectivity in two nearctic–neotropical songbirds: new insights from intrinsic markers. Ornithological Monographs, no. 61, pp 1–13
Cade BS, Richards JD (2005) User manual for Blossom statistical software. U.S. Geological Survey Open-File Report 2005-1353. www.fort.usgs.gov/products/software/blossom/blossom.asp
Calvert AM, Walde SJ, Taylor PD (2009) Nonbreeding-season drivers of population dynamics in seasonal migrants: conservation parallels across taxa. Avian Conservation and Ecology 4:5
Colwell MA (2010) Shorebird ecology, conservation, and management. University of California Press, Berkeley
Conklin JR, Battley PR, Potter MA, Ruthrauff DR (2011) Geographic variation in morphology of Alaska-breeding bar-tailed godwits (Limosa lapponica) is not maintained on their nonbreeding grounds in New Zealand. Auk 128:363–373
Degnan SM, Owens IPF, Clegg SM, Moritz CC, Kikkawa J (1999) MtDNA, microsatellites and coalescence: tracing the colonisation of Silvereyes through the southwest Pacific. In: Adams NJ, Slotow RH (eds) Proceedings of the 22nd international ornithological congress, Durban, BirdLife South Africa, Johannesburg, pp 1881–1898
Dunning JB Jr (1988) Significant encounters with marked birds. North Am Bird Bander 13:9
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fleischer RC, James HF, Olson SL (2008) Convergent evolution of Hawaiian and Australo-Pacific honeyeaters from distant songbird ancestors. Curr Biol 18:1927–1931
Flint PL, Meixell BW, Mallek EJ (2014) High fidelity does not preclude colonization: range expansion of molting black brant on the Arctic coast of Alaska. J Field Ornithol 85:75–83
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selections. Genetics 147:915–925
Gill RE Jr, McCaffery BJ, Tomkovich PS (2002) Wandering tattler (Heteroscelus incanus). In: Gill F, Poole A (eds) The birds of North America No. 642. The Birds of North America Inc., Philadelphia
Gill RE Jr, Douglas DC, Handel CM, Tibbitts TL, Hufford G, Piersma T (2014) Hemispheric-scale wind selection facilitates bar-tailed godwit circum-migration of the Pacific. Anim Behav 90:117–130
Gill RE Jr, Redmond RL (1992) Distribution and numbers of bristle-thighed curlews (Numenius tahitiensis) on Rangiroa Atoll. Notornis 39:17–26
Gill RE Jr, Butler RW, Tomkovich PS, Mundkur T, Handel C (1995) Conservation of North Pacific shorebirds. Wader Study Group Bulletin 77:82–91
Gill RE Jr, Handel CM, Ruthrauff DR (2013) Intercontinetal migratory connectivity and population structuring of Dunlins from western Alaska. Condor 115:525–534
Goudet J (1995) FSTAT (vers. 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Hancock JM (1999) Microsatellites and other simple sequences: genomic context and mutational mechanisms. In: Goldstein DB, Schlötterer C (eds) Microsatellites: evolution and applications. Oxford University Press, New York, pp 1–9
Handel CM, Dau CP (1988) Seasonal occurrence of migrant whimbrels and bristle-thighed curlews on the Yukon-Kuskokwim Delta, Alaska. Condor 90:782–790
Hasegawa M, Kishino H, Yano T (1985) Dating the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Hubisz MA, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:322–1332
Johnson OW, Fielding L, Fisher JP, Gold RS, Goodwill RH, Bruner AE, Furey JF, Brusseau PA, Brusseau NH, Johnson PM, Jukema J, Prince LL, Tenney MJ, Fox JW (2012) New insight concerning transoceanic migratory pathways of pacific golden-plovers (Pluvialis fulva): the Japan stopover and other linkages as revealed by geolocators. Wader Study Group Bull 119:1–8
Kingsford RT, Watson JEM, Lundquist CJ, Johnston EL, Atherton J, Gawel M, Keith DA, Mackey BG, Morley C, Possingham HP, Raynor B, Recher HF, Wilson KA (2009) Major conservation policy issues for biodiversity in Oceania. Conserv Biol 23:834–840
Legra L, Li X, Peterson AT (2008) Biodiversity consequences of sea level rise in New Guinea. Pac Conserv Biol 14:191–199
Longmire JL, Lewis AK, Brown NC, Buckingham JM, Clark LM, Jones MD, Meincke LJ, Meyne J, Ratliff RL, Ray FA, Wagner RP, Moyzis RK (1988) Isolation and molecular characterization of a highly polymorphic centromeric tandem repeat in the family Falconidae. Genomics 2:14–24
Marks JS, Redmond RL (1994) Conservation problems and research needs for bristle-thighed curlews Numenius tahitiensis on their wintering grounds. Bird Conserv Int 4:329–341
Marks JS, Redmond RL (1996) Demography of bristle-thighed curlews Numenius tahitiensis wintering on Laysan Island. Ibis 138:438–447
Marks JS, Tibbitts TL, Gill RE Jr, McCaffery BJ (2002) Bristle-thighed curlew (Numenius tahitiensis). In: Poole A (ed) The birds of North America Online No. 705. Cornell Lab of Ornithology, Ithaca
Meirmans PG (2006) Using the AMOVA framework to estimate a standardized genetic differentiation measure. Evolution 60:2399–2402
Minton C, Gosbell K, Johns P, Christie M, Fox JW, Afanasyev V (2010) Initial results from light level geolocator trials on ruddy turnstone Arenaria interpres reveal unexpected migration route. Wader Study Group Bull 117:9–14
Oosterhout CV, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol 4:535–538
Pearce JM, Blums P, Lindberg MS (2008) Site fidelity is an inconsistent determinant in population structure of the hooded merganser (Lophodytes cucullatus): evidence from genetic, mark-recapture, and comparative data. Auk 125:711–722
Piersma T, van Gils J, Wiersma P (1996) Family Scolopacidae (sandpipers, snipes and phalaropes). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world, hoatzin to auks, vol 3. Lynx Edicions, Barcelona, pp 444–487
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Pratt HD, Bruner PL, Berrett DG (1987) The birds of Hawaii and Tropical Pacific. Princeton University Press, Princeton
Primmer CR, Møller AP, Ellegren H (1995) Resolving genetic relationships with microsatellite markers: a parentage testing system for the swallow Hirundo rustica. Mol Ecol 4:493–498
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure from multilocus genotype data. Genetics 155:945–959
Quinn TW, Wilson AC (1993) Sequence evolution in and around the mitochondrial control region in birds. J Mol Evol 37:417–425
Rousset F (1996) Equilibrium values of measures of population subdivision for stepwise mutation processes. Genetics 142:1357–1362
Ruokonen M, Kvist L, Lumme J (2000) Close relatedness between mitochondrial DNA from seven Anser goose species. J Evol Biol 13:532–540
Schneider S, Roessli D, Excoffier L (2000) ARLEQUIN version 2.0: a software for population genetic data analysis. Genetics and Biometry Laboratory, University of Geneva, Geneva
Sonsthagen SA, Talbot SL, White CM (2004) Gene flow and genetic characterization of northern goshawks breeding in Utah. Condor 106:826–836
Sonsthagen SA, Coonan TJ, Latta BC, Sage GK, Talbot SL (2012) Genetic diversity of a newly established population of golden eagles on the Channel Islands, California. Biol Conserv 146:116–122
Steadman DW (1997) Human-caused extinction of birds. In: Reaka-Kudla ML, Wilson DE, Wilson EO (eds) Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press, Washington, D.C., pp 139–162
Tajima F (1989) The effect of change in population size on DNA polymorphism. Genetics 123:597–601
Thuman KA, Widemo F, Piertney SB (2002) Characterization of polymorphic microsatellite DNA markers in the ruff (Philomachus pugnax). Mol Ecol Notes 2:276–277
Topchy A, Scribner K, Punch W (2004) Accuracy-driven loci selection and assignment of individuals. Mol Ecol Notes 4:798–800
Van Treuren R (1998) Estimating null allele frequencies at a microsatellite locus in the oystercatcher (Haematopus ostralegus). Mol Ecol 7:1413–1417
Wang IJ (2010) Recognizing the temporal distinctions between landscape genetics and phylogeography. Mol Ecol 19:2605–2608
Webster MS, Marra PP (2005) The importance of understanding migratory connectivity and season interactions. In: Greenberg R, Marra PP (eds) Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press, Baltimore, pp 199–209
Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT (2002) Links between worlds: unraveling migratory connectivity. Trends Ecol Evol 17:76–83
Wennerberg L, Bensch T (2001) Geographic variation in the dunlin (Calidris alpina) as revealed by morphology, mtDNA and microsatellites. In: Genetic variation and migration in waders. Doctoral thesis, Lund University
Williams TC, Williams JM (1990) The orientation of transoceanic migrants. In: Gwinner E (ed) Bird migration: physiology and ecophysiology. Springer, Berlin, pp 7–21
Williams I, Guzzetti BM, Gust JR, Sage GK, Gill RE Jr, Tibbitts TL, Sonsthagen SA, Talbot SL (2012) Polymorphic microsatellite loci identified through development and cross-species amplification within shorebirds. J Ornithol 153:593–601
Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177–1191
Acknowledgments
Funding for this research was provided by the U.S. Geological Survey Alaska Science Center and Laboratories of Analytical Biology, National Museum of Natural History, Smithsonian Institution. Technological support was provided the University of Alaska Life Science Informatics computer cluster (NIH P20RR016466). Richard Lanctot, U.S. Fish and Wildlife Service, helped design the study and Sue Thomas, U.S. Fish and Wildlife Service, procured initial funding. We thank the James Dean, National Museum of Natural History, Smithsonian Institution, for subsampling specimens. George Sage and Judy Gust, U.S. Geological Survey, provided laboratory assistance. David Douglas, U.S. Geological Survey, provided advice on the spatial analysis. Comments by Robert Wilson, University of Alaska Fairbanks, John Pearce, U.S. Geological Survey, and three anonymous reviewers, improved the manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 3.
Rights and permissions
About this article
Cite this article
Sonsthagen, S.A., Tibbitts, T.L., Gill, R.E. et al. Spatial genetic structure of bristle-thighed curlews (Numenius tahitiensis): breeding area differentiation not reflected on the non-breeding grounds. Conserv Genet 16, 223–233 (2015). https://doi.org/10.1007/s10592-014-0654-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-014-0654-4