Abstract
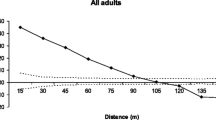
We assessed the pollen and seed dispersal patterns, genetic diversity, inbreeding and spatial genetic structure of Himatanthus drasticus (Apocynaceae), a tree native to the Brazilian Savanna (Cerrado) that is heavily exploited for its medicinal latex. The study was conducted in the Araripe National Forest, Ceará State, Brazil. Within a one-hectare plot, samples were collected from all adult trees, adult trees located in the immediate vicinity of the plot, and seedlings. All sampled individuals were mapped and genotyped using microsatellite markers. High levels of polymorphism and significant levels of inbreeding were found, which indicates that self-fertilisation and mating among relatives occur in this population. Both the adults and seedlings had significant spatial genetic structure up to ~40 m and our results confirmed the occurrence of isolation by distance. Pollen and seeds were dispersed over short distances and immigration of pollen and seeds into the plot was estimated at 13 and 9 %, respectively. Taking into consideration the degree of inbreeding, relatedness, intrapopulation spatial genetic structure and pollen dispersal distance, we recommend collecting seeds from a large number of trees spaced at least 150 m apart to avoid collecting seeds from related individuals and an overlap of pollen pools among seed trees.



Similar content being viewed by others
References
Amaro MS, Medeiros Filho S, Guimarães RM, Teófilo EM (2006) Morfologia de frutos, sementes e de plântulas de janaguba (Himatanthus drasticus (Mart.) Plumel.—Apocynaceae). Revista Brasileira de Sementes 28:63–71
Amorim FW, DeÁvila RS Jr, Camargo AJA, Vieira AL, Oliveira PE (2009) A hawkmoth crossroads? Species richness, seasonality and biogeographical affinities of Sphingidae in a Brazilian Cerrado. J Biogeogr 36:662–674
Assogbadjo AE, Kyndt T, Sinsin B, Gheysen G, Van Damme P (2006) Patterns of genetic and morphometric diversity in baobab (Adansonia digitata) populations across different climatic zones of Benin (West Africa). Ann Bot 97:819–830
Augspurger CK, Kelly CK (1984) Pathogen mortality of tropical tree seedlings: experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 61:211–217
Bacles CFE, Lowe AJ, Ennos RA (2006) Effective seed dispersal across a fragmented landscape. Science 311:628–700
Baldauf C, Ciampi MB, Vigna BBZ, Mori GM, Guedes JPP, Souza AP, Santos FAM (2011) Characterization of microsatellite loci in Himatanthus drasticus (Apocynaceae), a medicinal plant from the Brazilian savanna. Am J Bot 98:e244–e246
Baldauf C, Ciampi-Guillardi M, Sebbenn AM, Santos FAM, Souza AP (2013) Tapping latex and alleles? The impacts of bark and latex harvesting on the genetic diversity of Himatanthus drasticus (Apocynaceae). For Ecol Manage 310:434–441
Bawa KS (1990) Plant–pollinator interactions in tropical rain forests. Annu Rev Ecol Syst 21:399–422
Bennett AF, Radford JQ, Haslem A (2006) Properties of land mosaics: implications for nature conservation in agricultural environments. Biol Conserv 133:250–264
Burczyk J, Adams WT, Shimizu JY (1996) Mating patterns and pollen dispersal in a natural knobcone pine (Pinus attenuate Lemmon.) stand. Heredity 77:251–260
Cavalcanti AC, Lopes OF (1994) Condições edafoclimáticas da Chapada do Araripe e viabilidade de produção sustentável de culturas. EMBRAPA-SPI, Brasilia
Collevatti RG, Grattapaglia D, Hay JD (2001) Population genetic structure of the endangered tropical tree species Caryocar brasiliense, based on variability at microsatellite loci. Mol Ecol 10:349–356
Collevatti RG, Lima JS, Soares TN, Telles MPC (2010) Spatial genetic structure and life history traits in Cerrado tree species: inferences for conservation. Braz J Nat Conserv 8:54–59
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
Diniz IR, Morais HC, Botelho AMF, Venturoli F, Cabral BC (1999) Lepidopteran caterpillar fauna on lactiferous host plants in the central Brazilian cerrado. Rev Bras Biol 59:627–635
Dow BD, Ashley MV (1996) Microsatellite analysis of seed dispersal and parentage of sampling in bur oak, Quercus macrocarpa. Mol Ecol 5:615–627
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Arginia spinosa (L.) Skeels] endemic to Morroco. Theor Appl Genet 92:832–839
Felfili JM, Silva-Junior MC (1988) Distribuição dos diâmetros numa faixa de cerrado na Fazenda Água Limpa (FAL) em Brasilia-DF. Acta Botanica Brasilica 2:85–104
Fonseca MG, Martini AMZ, Santos FAM (2004) Spatial structure of Aspidosperma polyneuron in two semi-deciduous forests in southeast Brazil. J Veg Sci 15:41–48
Frankel OH, Soulé ME (1981) Conservation and evolution. Cambridge University Press, Cambridge
Frankham R (2004) Resolving the genetic paradox in invasive species. Heredity 94:385–400
Gaino APSC, Silva AM, Moraes MA, Alves PF, Moraes MLT, Freitas MLM, Sebbenn AM (2010) Understanding the effects of isolation on seed and pollen flow, spatial genetic structure and effective population size of the dioecious tropical tree species Myracrodruon urundeuva. Conserv Genet 11:1631–1643
Goudet J (1995) FSTAT (version 1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2(4):618–620
Hardy OJ, Maggia L, Bandou E, Breyne P, Caron J, Chevallier MH, Doligez A, Dutech C, Kremer A, Latouche-Hallé C, Troispoux V, Veron V, Degen B (2006) Fine-scale genetic structure and gene dispersal inferences in 10 Neotropical tree species. Mol Ecol 15:559–571
Hutchings MJ (1997) The Structure of Plant Populations. In: Crawley MJ (ed) Plant ecology. Blackwell, Oxford, pp 325–358
Iwaizumi MG, Takahashi M, Watanabe A, Ubukata M (2009) Simultaneous evaluation of paternal and maternal immigrant gene flow and the implications for the overall genetic composition of Pinus densiflora Dispersed Seeds. J Hered 101:144–153
Jones FA, Chen J, Weng GJ, Hubbell SP (2005) A genetic evaluation of seed dispersal in the neotropical tree Jacaranda copaia (Bignoniaceae). Am Nat 166:543–555
Jump AS, Peñuelas J (2006) Genetic effects of chronic habitat fragmentation in a wind-pollinated tree. Proc Natl Acad Sci USA 103:8096–8100
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kanashiro M, Thompson IS, Yared JAG, Loveless MD, Coventry P, Martins-da-Silva RCV, Degen B, Amaral W (2002) Improving conservation values of managed forests: the Dendrogene Project in the Brazilian Amazon. Unasylva 209:25–33
Konnert M, Maurer W, Degen B, Kätzel R (2011) Genetic monitoring in forests—early warning and controlling system for ecosystemic changes. iForest Biogeosci For 4:77–81
Köppen W (1948) Climatologia: con un estudio de los climas de la tierra. Fondo de Cultura Econômica: 479
Lacerda AEB, Kanashiro M, Sebbenn AM (2008) Effects of Reduced Impact Logging on genetic diversity and spatial genetic structure of a Hymenaea courbaril population in the Brazilian Amazon Forest. For Ecol Manage 255:1034–1043
Laikre L (2010) Genetic diversity is overlooked in international conservation policy implementation. Conserv Genet 11:349–354
Laikre L, Nilsson T, Primmer CR, Ryman N, Allendorf FW (2009) Importance of genetics in the interpretation of favourable conservation status. Conserv Biol 23:1378–1381
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Lander TA, Boshier DH, Harris SA (2010) Fragmented but not isolated: contribution of single trees, small patches and long-distance pollen flow to genetic connectivity for Gomortega keule, an endangered Chilean tree. Biol Conserv 143:2583–2590
Levin DA, Kerster HW (1974) Gene flow in seed plants. Evol Biol 7:r220
Liede S, Weberling F (1995) On the inflorescence structure of the Asclepiadaceae. Plant Syst Evol 197:99–109
Lorenzi H, de Abreu Matos FJ (2002) Plantas medicinais no Brasil: nativas e exóticas, 2nd edn. Instituto Plantarum, Nova Odessa
Manoel R, Alves P, Dourado C, Gaino A, Freitas M, Moraes M, Sebbenn A (2011) Contemporary pollen flow, mating patterns and effective population size inferred from paternity analysis in a small fragmented population of the Neotropical tree Copaifera langsdorffii Desf. (Leguminosae-Caesalpinioideae). Conserv Genet 13:613–623
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood based paternity inference in natural populations. Mol Ecol 7:639–655
Meagher TR (1986) Analysis of paternity within a natural population of Chamaelirium luteum. I. Identification of most-likely male parents. Am Nat 128:199–215
Meagher TR, Thompson E (1987) Analysis of parentage for naturally established seedlings of Chamaelirim luteum (Liliaceae). Ecology 68:803–812
Milfont CID (2011) Estrutura espacial de populações de janaguba (Himatanthus drasticus (Mart) Plumel) na Chapada do Araripe, Ceará. Monography (Bach). Universidade Regional do Cariri, Brazil
Moraes MLT, Sebbenn AM (2010) Pollen dispersal between isolated trees in the Brazilian savannah: a case study of the Neotropical tree Hymenaea stigonocarpa. Biotropica 43:192–199
Mousinho KC, Oliveira CC, Ferreira JRO, Carvalho AA, Magalhães HIF, Bezerra DP, Alves APNN, Costa-Lotufo LV, Pessoa C, De Matos MPV (2011) Antitumor effect of laticifer proteins of Himatanthus drasticus (Mart.) Plumel-Apocynaceae. J Ethnopharmacol 137:421–426
Oliveira PS, Marquis RJ (2002) The cerrados of Brazil: ecology and natural history of a neotropical savanna. Columbia Univ Press, New York
Pandey M, Rajora OP (2012) Higher fine-scale genetic structure in peripheral than core populations of a long-lived and mixed-mating conifer-eastern white cedar (Thuja occidentalis L.). Biochem Syst 12:48
Petit RJ, Duminil J, Fineschi S, Hampe A, Salvini D, Vendramin GG (2005) Invited review: comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Mol Ecol 14:689–701
Plumel MM (1991) Le genre Himatanthus (Apocynaceae) révision taxonomique: Bradea
Rathmacher G, Niggemann M, Köhnen M, Ziegenhagen B, Bialozyt R (2010) Short-distance gene flow in Populus nigra L. accounts for small-scale spatial genetic structures: implications for in situ conservation measures. Conserv Genet 11:1327–1338
Rebouças SO, Grivicich I, Santos MS, Rodriguez P, Gomes MD, Oliveira SQ, da Silva J, Ferraz ABF (2011) Antiproliferative effect of a traditional remedy, Himatanthus articulatus bark, on human cancer cell lines. J Ethnopharmacol 137:926–929
Rijkers T, Ogbazghi W, Wessel M, Bongers F (2006) The effect of tapping for frankincense on sexual reproduction in Boswellia papyrifera. J Appl Ecol 43:1188–1195
Rosas F, Quesada M, Lobo JA, Sork VL (2011) Effects of habitat fragmentation on pollen flow and genetic diversity of the endangered tropical tree Swietenia humilis (Meliaceae). Biol Conserv 144:3082–3088
Sant’Anna CS, Sebbenn AM, Klabunde GHF, Bittencourt R, Nodari RO, Mantovani A, Reis MS (2013) Realized pollen and seed dispersal within a continuous population of the dioecious coniferous Brazilian pine [Araucaria angustifolia (Bertol.) Kuntze]. Conserv Genet 14:601–613
Santos FAM (2001) MAPA. UNICAMP, Campinas
Schlindwein C, Darrault RO, Grisi T (2004) Reproductive strategies in two sphingophilous apocynaceous trees attracting pollinators through nectar or deceit. In: Proceedings of the 2nd Symposium of the A. F. W. Schimper-Foundation, Stuttgart, pp 215–227
Sebbenn AM, Carvalho ACM, Freitas MLM, Moraes SMB, Gaino APSC, Silva JM, Jolivet C, Moraes MLT (2011) Low levels of realized seed and pollen gene flow and strong spatial genetic structure in a small, isolated and fragmented population of the tropical tree Copaifera langsdorffii Desf. Heredity 106:134–145
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. Freeman, New York
Soons MB, Heil GW, Nathan R, Katul GG (2004) Determinants of long-distance seed dispersal by wind in grasslands. Ecology 85:3056–3068
Stewart K (2009) Effects of bark harvest and other human activity on populations of the African cherry (Prunus africana) on Mount Oku, Cameroon. For Ecol Manage 258:1121–1128
Tarazi R, Mantovani A, Reis MS (2010) Fine-scale spatial genetic structure and allozymic diversity in natural populations of Ocotea catharinensis Mez. (Lauraceae). Conserv Genet 11:965–976
Tarazi R, Sebbenn AM, Kageyama PY, Vencovsky R (2013) Edge effects enhance selfing and seed harvesting efforts in the insect-pollinated Neotropical tree Copaifera langsdorffii (Fabaceae). Heredity 110:578–585
Vekemans X, Hardy OJ (2004) New insights from fine-scale spatial genetic structure analyses in plant populations. Mol Ecol 13:921–935
Wright S (1943) Isolation by distance. Genetics 28:114
Zardo R, Henriques R (2011) Growth and fruit production of the tree Caryocar brasiliense in the Cerrado of central Brazil. Agrofor Syst 82:15–23
Zucchi MI, Brondani RPV, Pinheiro JB, Chaves LJ, Coelho ASG, Vencovsky R (2003) Genetic structure and gene flow in Eugenia dysenterica DC in the Brazilian Cerrado utilizing SSR markers. Genet Mol Biol 26:449–457
Acknowledgments
The authors thank D. Pessoa and the staff of FLONA Araripe for their support during fieldwork. We are also grateful to T. Eugênio and P. Zambon for technical support in the laboratory. This work was supported by the National Counsel of Technological and Scientific Development through a research grant [grant number 472127/2008-0], a PhD fellowship granted to C.B. and research productivity fellowships granted to A.M.S., A.P.S. and F.A.M.S. and by the São Paulo Research Foundation through a research grant [grant number 2008/08737-4], an undergraduate scholarship to T.J.A. and a post-doctoral fellowship to M.B.C. We would also like to thank A. G. Young and two anonymous reviewers for their important suggestions in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldauf, C., Ciampi-Guillardi, M., Aguirra, T.J. et al. Genetic diversity, spatial genetic structure and realised seed and pollen dispersal of Himatanthus drasticus (Apocynaceae) in the Brazilian savanna. Conserv Genet 15, 1073–1083 (2014). https://doi.org/10.1007/s10592-014-0600-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-014-0600-5