Abstract
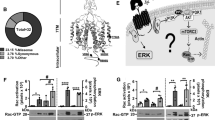
Stanniocalcin-l (STC-1) is a secreted glycoprotein hormone that regulates calcium and phosphate homeostasis. STC-1 expression is upregulated in several cancers including breast cancer, and has been shown to be prognostic. Although these clinical observations implicate STC-1 as a potential tumor marker, it is still unclear whether STC-1 confers a malignant phenotype. In this study, this question was addressed by overexpressing STC-1 in the human breast cancer cell line MDA-MB-231 and examining the resultant phenotype in vitro and in vivo. Overexpression of STC-1 enhanced invasiveness of MDA-MB-231 cells in vitro and promoted their lung metastasis in vivo, while having no effect on proliferation, adhesion, or proteinase activity. The addition of soluble STC-1 to MDA-MB-231 cultures resulted in the activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, suggesting a mechanistic basis for the observed increases in cell motility and metastasis. Taken together, it was indicated that secreted STC-1 promotes metastatic potential of breast cancer cells via activation of PI3K/AKT.





Similar content being viewed by others
Abbreviations
- STC-1:
-
Stannocalcin-1
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphoinositide 3-kinase
References
Wagner GF, Hammpong M, Park CM, Copp DH (1986) Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen Comp Endocrinol 63(3):481–491
Chang AC, Janosi J, Hulsbeek M, de Jong D, Jeffrey KJ, Noble JR, Reddel RR (1995) A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol 112(2):241–247
Chang AC, Jeffrey KJ, Tokutake Y, Shimamoto A, Neumann AA, Dunham MA, Cha J, Sugawara M, Furuichi Y, Reddel RR (1998) Human stanniocalcin (STC): genomic structure, chromosomal localization, and the presence of CAG trinucleotide repeats. Genomics 47(3):393–398
Jellinek DA, Chang AC, Larsen MR, Wang X, Robinson PJ, Reddel RR (2000) Stanniocalcin 1 and 2 are secreted as phosphoproteins from human fibrosarcoma cells. Biochem J 350:453–461
Olsen HS, Cepeda MA, Zhang QQ, Rosen CA, Vozzolo BL, Wagner GF (1996) Human stanniocalcin: a possible hormonal regulator of mineral metabolism. Proc Natl Acad Sci USA 93(5):1792–1796
Fujiwara Y, Sugita Y, Nakamori S, Miyamoto A, Shiozaki K, Nagano H, Sakon M, Monden M (2000) Assessment of Stanniocalcin-1 mRNA as a molecular marker for micrometastases of various human cancers. Int J Oncol 16(4):799–804
Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, Bast RC, Lin SH, Liu J (2010) Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst 102(11):812–827
Tohmiya Y, Koide Y, Fujimaki S, Harigae H, Funato T, Kaku M, Ishii T, Munakata Y, Kameoka J, Sasaki T (2004) Stanniocalcin-1 as a novel marker to detect minimal residual disease of human leukemia. Tohoku J Exp Med 204(2):125–133
Tamura S, Oshima T, Yoshihara K, Kanazawa A, Yamada T, Inagaki D, Sato T, Yamamoto N, Shiozawa M, Morinaga S, Akaike M, Kunisaki C, Tanaka K, Masuda M, Imada T (2011) Clinical significance of STC1 gene expression in patients with colorectal cancer. Anticancer Res 31(1):325–330
Du YZ, Gu XH, Li L, Gao F (2011) The diagnostic value of circulating stanniocalcin-1 mRNA in non-small cell lung cancer. J Surg Oncol 104(7):836–840
Shirakawa M, Fujiwara Y, Sugita Y, Moon JH, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Mori M, Doki Y (2012) Assessment of Stanniocalcin-1 as a prognostic marker in human esophageal squamous carcinoma. Oncol Rep 27(4):940–946
Joensuu K, Heikkila P, Andersson LC (2002) Tumor dormancy: elevated expression of stanniocalcins in late relapsing breast cancer. Cancer Lett 265(1):76–83
Wascher RA, Huynh KT, Giuliano AE, Hansen NM, Singer FR, Elashoff D, Hoon DS (2003) Stanniocalcin-1: a novel molecular blood and bone marrow marker for human breast cancer. Clin Cancer Res 9(4):1427–1435
Bautista S, Vallès H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C (1998) In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res 4(12):2925–2929
Courjal F, Cuny M, Simony-Lafontaine J, Louason G, Speiser P, Zeillinger R, Rodriguez C, Theillet C (1997) Mapping of DNA amplifications at 15 chromosomal localizations in 1875 breast tumors: definition of phenotypic groups. Cancer Res 57(19):4360–4367
Pignatelli J, LaLonde SE, LaLonde DP, Clarke D, Turner CE (2012) Actopaxin (α-parvin) phosphorylation is required for matrix degradation and cancer cell invasion. J Biol Chem 287(44):37309–37320
Kestler DP, Foster JS, Bruker CT, Prenshaw JW, Kennel SJ, Wall JS, Weiss DT, Solomon A (2011) ODAM expression inhibits human breast cancer tumorigenesis. Breast Cancer (Auckl) 5:73–85
Roy S, Chakravarty D, Cortez V, De Mukhopadhyay K, Bandyopadhyay A, Ahn JM, Raj GV, Tekmal RR, Sun L, Vadlamudi RK (2012) Significance of PELP1 in ER-negative breast cancer metastasis. Mol Cancer Res 10(1):25–33
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436(7050):518–524
Tanaka M, Kogawa K, Nishihori Y, Kuribayashi K, Nakamura K, Muramatsu H, Koike K, Sakamaki S, Niitsu Y (1997) Suppression of intracellular Cu-Zn SOD results in enhanced motility and metastasis of Meth A sarcoma cells. Int J Cancer 73(2):187–192
He LF, Wang TT, Gao QY, Zhao GF, Huang YH, Yu LK, Hou YY (2011) Stanniocalcin-1 promotes tumor angiogenesis through up-regulation of VEGF in gastric cancer cells. J Biomed Sci 18:39
Ohkouchi S, Block GJ, Katsha AM, Kanehira M, Ebina M, Kikuchi T, Saijo Y, Nukiwa T, Prockop DJ (2012) Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther 20(2):417–423
Peña C, Céspedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, Hägglöf C, Birgisson H, Bojmar L, Jirström K, Sandström P, Olsson E, Veerla S, Gallardo A, Sjöblom T, Chang AC, Reddel RR, Mangues R, Augsten M, Ostman A (2013) STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer Res 73(4):1287–1297
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3(5):362–374
Sotsios Y, Ward SG (2000) Phosphoinositide 3-kinase: a key biochemical signal for cell migration in response to chemokines. Immunol Rev 177:217–235
Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y (2010) Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29(35):4947–4958
Kuribayashi K, Nakamura K, Tanaka M, Sato T, Kato J, Sasaki K, Takimoto R, Kogawa K, Terui T, Takayama T, Onuma T, Matsunaga T, Niitsu Y (2007) Essential role of protein kinase C zeta in transducing a motility signal induced by superoxide and a chemotactic peptide, fMLP. J Cell Biol 176(7):1049–1060
Luo CW, Kawamura K, Klein C, Hsueh AJ (2004) Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: mediation through specific STC1 receptors. Mol Endocrinol 18(8):2085–2096
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research in Japan (Grant No. 24590700) M. T., K. K., D. K., and N. W.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
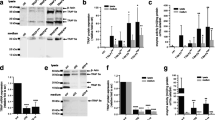
Real-time PCR and western blotting analysis of STC-1 expression in MDA-MB-231 breast cancer cell lines. (a) Transcript and (b) protein expression of STC-1 was detected by Real-time PCR and western blotting, respectively, in MB231-M and -S cells stably transfected with an empty control vector and a vector encoding secreted STC-1, respectively. Transcript expression was determined relative to the internal control GAPDH and Ran was used as a loading control for the western blotting. Supplementary material 1 (TIFF 19762 kb)
Supplementary Figure 2
The expression of phosphorylated Akt in MB231-M and -S cell lysates. Whole cell lysate from MB231-M and -S that cultured without FBS for 24 h was harvested. The level of phosphorylated Akt was detected by western blotting as described above. Supplementary material 2 (TIFF 19762 kb)
Rights and permissions
About this article
Cite this article
Murai, R., Tanaka, M., Takahashi, Y. et al. Stanniocalcin-1 promotes metastasis in a human breast cancer cell line through activation of PI3K. Clin Exp Metastasis 31, 787–794 (2014). https://doi.org/10.1007/s10585-014-9668-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-014-9668-z