Abstract
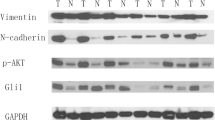
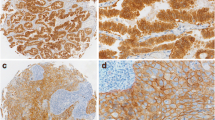
The five-year survival rate in advanced non-small cell lung cancer (NSCLC) remains below ten percent. The invasive and metastatic nature of NSCLC tumor cells contributes to the high mortality rate, and as such the mechanisms that govern NSCLC metastasis is an active area of investigation. Two surface receptors that influence NSCLC invasion and metastasis are the hepatocyte growth factor receptor (HGFR/MET) and fibroblast growth factor-inducible 14 (FN14). MET protein is over-expressed in NSCLC tumors and associated with poor clinical outcome and metastasis. FN14 protein is also elevated in NSCLC tumors and positively correlates with tumor cell migration and invasion. In this report, we show that MET and FN14 protein expressions are significantly correlated in human primary NSCLC tumors, and the protein levels of MET and FN14 are elevated in metastatic lesions relative to patient-matched primary tumors. In vitro, HGF/MET activation significantly enhances FN14 mRNA and protein expression. Importantly, depletion of FN14 is sufficient to inhibit MET-driven NSCLC tumor cell migration and invasion in vitro. This work suggests that MET and FN14 protein expressions are associated with the invasive and metastatic potential of NSCLC. Receptor-targeted therapeutics for both MET and FN14 are in clinical development, the use of which may mitigate the metastatic potential of NSCLC.







Similar content being viewed by others
References
Jemal A et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Heist RS, Engelman JA (2012) SnapShot: non-small cell lung cancer. Cancer Cell 21(3):448 e2
Stella GM, Benvenuti S, Comoglio PM (2010) Targeting the MET oncogene in cancer and metastases. Expert Opin Investig Drug 19(11):1381–1394
Tsuta K et al (2012) c-MET/phospho-MET protein expression and MET gene copy number in non-small cell lung carcinomas. J Thorac Oncol 7(2):331–339
Yang Y et al (2008) A selective small molecule inhibitor of c-Met, PHA-665752, reverses lung premalignancy induced by mutant K-ras. Mol Cancer Ther 7(4):952–960
Bean J et al (2007) MET amplification occurs with or without T790 M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 104(52):20932–20937
Benedettini E et al (2010) Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol 177(1):415–423
Navab R et al (2009) Co-overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI-H460 non-small cell lung carcinoma cells. Neoplasia 11(12):1292–1300
Feng SL et al (2000) The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 156(4):1253–1261
Tran NL et al (2003) The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol 162(4):1313–1321
Tran NL et al (2006) Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res 66(19):9535–9542
Watts GS et al (2007) Identification of Fn14/TWEAK receptor as a potential therapeutic target in esophageal adenocarcinoma. Int J Cancer 121(10):2132–2139
Willis AL et al (2008) The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res 6(5):725–734
Whitsett TG et al (2012) Elevated expression of Fn14 in non-small cell lung cancer correlates with activated EGFR and promotes tumor cell migration and invasion. Am J Pathol 181(1):111–120
Fortin SP et al (2009) Tumor necrosis factor-like weak inducer of apoptosis stimulation of glioma cell survival is dependent on Akt2 function. Mol Cancer Res 7(11):1871–1881
Tran NL et al (2005) The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J Biol Chem 280(5):3483–3492
Zhou H et al (2011) Development and characterization of a potent immunoconjugate targeting the Fn14 receptor on solid tumor cells. Mol Cancer Ther 10(7):1276–1288
Knudsen BS et al (2009) A novel multipurpose monoclonal antibody for evaluating human c-Met expression in preclinical and clinical settings. Appl Immunohistochem Mol Morphol 17(1):57–67
Jackson EL et al (2001) Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15(24):3243–3248
Bardeesy N et al (2002) Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419(6903):162–167
DuPage M, Dooley AL, Jacks T (2009) Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 4(7):1064–1072
Nakayama M et al (2003) Fibroblast growth factor-inducible 14 mediates multiple pathways of TWEAK-induced cell death. J Immunol 170(1):341–348
Fortin Ensign SP et al (2013) The Src homology 3 domain-containing guanine nucleotide exchange factor is overexpressed in high-grade gliomas and promotes tumor necrosis factor-like weak inducer of apoptosis-fibroblast growth factor-inducible 14-induced cell migration and invasion via tumor necrosis factor receptor-associated factor 2. J Biol Chem 288(30):21887–21897
Chuang YY et al (2004) Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res 64(22):8271–8275
Ji H et al (2007) LKB1 modulates lung cancer differentiation and metastasis. Nature 448(7155):807–810
Chen Z et al (2012) A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483(7391):613–617
Franks LM et al (1976) Metastasizing tumors from serum-supplemented and serum-free cell lines from a C57BL mouse lung tumor. Cancer Res 36(3):1049–1055
Layton MG, Franks LM (1984) Heterogeneity in a spontaneous mouse lung carcinoma: selection and characterisation of stable metastatic variants. Br J Cancer 49(4):415–421
Birchmeier C et al (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4(12):915–925
Gherardi E et al (2012) Targeting MET in cancer: rationale and progress. Nat Rev Cancer 12(2):89–103
Webb CP et al (1998) Evidence for a role of Met-HGF/SF during Ras-mediated tumorigenesis/metastasis. Oncogene 17(16):2019–2025
Matteucci E, Bendinelli P, Desiderio MA (2009) Nuclear localization of active HGF receptor Met in aggressive MDA-MB231 breast carcinoma cells. Carcinogenesis 30(6):937–945
Winkles JA (2008) The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7(5):411–425
Sanz-Pamplona R et al (2011) Expression of endoplasmic reticulum stress proteins is a candidate marker of brain metastasis in both ErbB-2 + and ErbB-2- primary breast tumors. Am J Pathol 179(2):564–579
Wang J et al (2013) Clinical correlations and prognostic relevance of Fn14 expression in breast carcinoma. Histol Histopathol 28(7):859–864
Eder JP et al (2009) Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15(7):2207–2214
Zhou H et al (2013) The TWEAK receptor Fn14 is a therapeutic target in melanoma: immunotoxins targeting Fn14 receptor for malignant melanoma treatment. J Invest Dermatol 133(4):1052–1062
Acknowledgments
We would like to thank Serdar Tuncali, Andrew Nelson, Irene Cherni, and Guy Raz for technical assistance and Drs. Hideo Yagita and Jennifer Michaelson (Biogen Idec) for providing the FN14 monoclonal antibody. This work was supported in part by the NIH grant, R01 CA130940 (N.L.T.), and grants from the St, Joseph’s Foundation and American Lung Association, RG-224607-N (L.J.I.).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Whitsett, T.G., Fortin Ensign, S.P., Dhruv, H.D. et al. FN14 expression correlates with MET in NSCLC and promotes MET-driven cell invasion. Clin Exp Metastasis 31, 613–623 (2014). https://doi.org/10.1007/s10585-014-9653-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-014-9653-6