Abstract
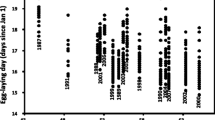
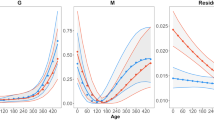
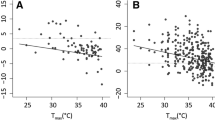
A shift in climatic conditions may directly modify critical organismal traits (such as reproductive output and offspring phenotypes), and experimental studies to document such direct effects thus may clarify the impacts of climate change on the species involved. The endangered Blue Mountains Water Skink (Eulamprus leuraensis) exhibits several traits predicted to imperil it under climate change: ectothermy, low reproductive output, specialisation to a restricted habitat type, montane endemicity, and a small geographic range. Congeneric species exhibit temperature-dependent sex determination, increasing potential sensitivity to climate change. We maintained wild-caught female lizards throughout pregnancy under thermal conditions simulating a shift in basking-time availability (3 vs 7 h/day) as might occur under climate change. Females with longer basking opportunities per day gave birth 2 weeks earlier, to slightly smaller offspring, that grew much faster in the first few weeks of life. Importantly, offspring sex ratios were not affected by maternal thermal regimes. Hence, some traits (e.g., offspring size, growth rates, dates of birth) are sensitive to ambient thermal conditions whereas other traits (e.g., offspring sex ratio and sprint speed) are not. On balance, the greatest threat to population persistence for E. leuraensis under climate change is likely to involve indirect effects mediated via habitat degradation (especially, drying-out of the hanging swamps) rather than direct thermal effects on lizard reproductive output or offspring phenotypes.
Similar content being viewed by others
References
Aubret F, Shine R (2010) Thermal plasticity in young snakes: how will climate change affect the thermoregulatory tactics of ectotherms? J Exp Biol 213:242–248
Beaumont LJ, Pitman AJ, Poulsen M, Hughes L (2007) Where will species go? Incorporating new advances in climate modelling into projections of species distributions. Glob Chang Biol 13:1368–1385
Benton TG, Plaistow SJ, Beckerman AP, Lapsley CT, Littlejohns S (2005) Changes in maternal investment in eggs can affect population dynamics. Proc Biol Sci 272:1351–1356
Borges PA (1999) Maternal thermoregulation and its consequences for offspring fitness in the Australian eastern water skink (Eulamprus quoyii). Ph.D. Thesis, The University of Sydney. p 123
Brown GP, Shine R (2006) Effects of nest temperature and moisture on phenotypic traits of hatchling snakes (Tropidonophis mairii, Colubridae) from tropical Australia. Biol J Linn Soc 89:159–168
Caley MJ, Schwarzkopf L (2004) Complex growth rate evolution in a latitudinally widespread species. Evolution 58:862–869
Cogger HG (2000) Reptiles and amphibians of Australia. 6th edn. Reed New Holland, Sydney
Deeming DC (2004) Post-hatching phenotypic effects of incubation in reptiles. In: Deeming D (ed) Reptile incubation. Nottingham Univ. Press, Nottingham, pp 229–251
Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672
Dirnbock T, Dullinger S, Grabherr G (2003) A regional impact assessment of climate and land-use change on alpine vegetation. J Biogeogr 30:401–417
Doughty P, Shine R (1997) Detecting life history trade-offs: measuring energy stores in “capital” breeders reveals costs of reproduction. Oecologia 110:508–513
Du W, Elphick M, Shine R (2010) Thermal regimes during incubation do not affect mean selected temperatures of hatchling lizards (Bassiana duperreyi, Scincidae). J Therm Biol 35:47–51
Dubey S, Chevalley M, Shine R (2010) Sexual dimorphism and sexual selection in a montane scincid lizard (Eulamprus leuraensis). Austral Ecology. doi:10.1111/j.1442–9993.2010.02119.x
Elphick MJ, Shine R (1998) Longterm effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards (Bassiana duperreyi, Scincidae). Biol J Linn Soc 63:429–447
Elphick MJ, Shine R (1999) Sex differences in optimal incubation temperatures in a scincid lizard species. Oecologia 118:431–437
Flatt T, Shine R, Borges-Landaez PA, Downes SJ (2001) Phenotypic variation in an oviparous montane lizard (Bassiana duperreyi): the effects of thermal and hydric incubation environments. Biol J Linn Soc 74:339–350
Forcada J, Trathan PN, Murphy EJ (2008) Life history buffering in Antarctic mammals and birds against changing patterns of climate and environmental variation. Glob Chang Biol 14:2473–2488
Gallagher RV, Hughes L, Leishman MR (2009) Phenological trends among Australian alpine species: using herbarium records to identify climate-change indicators. Aust J Bot 57:1–9
Greer AE (1989) The biology and evolution of Australian lizards. Surrey Beatty & Sons, Australia
Harlow PS (1996) A harmless technique for sexing hatchling lizards. Herpetol Rev 27:71–72
Harlow P, Shine R (1999) Temperature-dependent sex determination in the frillneck lizard, Chlamydosaurus kingii (Agamidae). Herpetologica 55:205–212
Harlow PS, Taylor JE (2000) Reproductive ecology of the jacky dragon (Amphibolurus muricatus): an agamid lizard with temperature-dependent sex determination. Austral Ecol 25:640–652
Huey RB, Tewksbury JJ (2009) Can behavior douse the fire of climate warming? Proc Natl Acad Sci USA 106:3647–3648
Hughes L (2000) Biological consequences of global warming: is the signal already apparent? Trends Ecol Evol 15:56–62
Hughes L (2002) Indicators of climate change. In: Howden M, Hughes L, Dunlop M, Zethoven I, Hilbert D, Chilcott C (eds) Climate change impacts on biodiversity in Australia. Commonwealth of Australia, Canberra, pp 58–62
Ji X, Lin LH, Lin CX, Qiu QB, Du Y (2006a) Sexual dimorphism and female reproduction in the many-lined sun skink (Mabuya multifasciata) from China. J Herpetol 40:351–357
Ji X, Lin LH, Luo LG, Gao JF, Han J (2006b) Gestation temperature affects sexual phenotype, morphology, locomotor performance, and growth of neonatal brown forest skinks, Sphenomorphus indicus. Biol J Linn Soc 88:453–463
Jiguet F, Gadot AS, Julliard R, Newson SE, Couvet D (2007) Climate envelope, life history traits and the resilience of birds facing global change. Glob Chang Biol 13:1672–1684
Kearney M, Shine R (2004) Developmental success, stability, and plasticity in closely related parthenogenetic and sexual lizards (Heteronotia, Gekkonida). Evolution 58:1560–1572
Kearney MR, Porter W, Shine R (2009) The potential for behavioral thermoregulation to buffer ‘cold-blooded’ animals against climate warming. Proc Natl Acad Sci USA 106:3835–3840
Kerr RA (2009) Global warming: Arctic summer sea ice could vanish soon but not suddenly. Science 323:1655
Langkilde T, Shine R (2005) Different optimal offspring sizes for sons versus daughters may favor the evolution of temperature-dependent sex determination in viviparous lizards. Evolution 59:2275–2280
LeBreton M (1996) Habitat and distribution of the Blue Mountains swamp skink (Eulamprus leuraensis). Honours degree. University of New South Wales, Sydney, p 46
Leips J, Richardson JML, Rodd FH, Travis J (2008) Conspecific density in two populations of the least kikkifish, Heterandria formosa. Evolution 63:1341–1347
Lourdais O, Shine R, Bonnet X, Guillon M, Naulleau G (2004) Climate affects offspring phenotypes in a viviparous snake. Oikos 104:551–560
McKinney ML (1997) Extinction vulnerability and selectivity: combining ecological and paleontological views. Ann Rev Ecolog Syst 28:495–516
Parmesan C (1996) Climate and species’ range. Nature 383:765–766
Phillips BL, Chipperfield JD, Kearney MR (2008) The toad ahead: challenges of modelling the range and spread of an invasive species. Wildl Res 35:222–234
Qualls FJ, Shine R (1998) Geographic variation in lizard phenotypes: importance of the incubation environment. Biol J Linn Soc 64:477–491
Radder RS, Quinn AE, Georges A, Sarre SD, Shine R (2008) Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol Lett 4:176–178
Robert KA, Thompson MB (2001) Sex determination: viviparous lizard selects sex of embryos. Nature 412:698–699
SAS Institute Inc. (2007) JMP. Version 7.0. SAS Institute, Cary, NC
Shea GM, Peterson M (1984) The Blue Mountains water skink, Sphenomorphus leuraensis (Lacertilia: Scincidae): a redescription, with notes on its natural history. Proc Linn Soc NSW 108:142–148
Shine R (1999) Egg-laying reptiles in cold climates: determinants and consequences of nest temperatures in montane lizards. J Evol Biol 12:918–926.
Shine R (2004a) Adaptive consequences of developmental plasticity. In: Deeming C (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham University Press, Nottingham, pp 187–210
Shine R (2004b) Seasonal shifts in nest temperature can modify the phenotypes of hatchling lizards, regardless of overall mean incubation temperature. Funct Ecol 18:43–49
Shine R (2006) Is increased maternal basking an adaptation or a pre-adaptation to viviparity in lizards? J Exp Zool 305A:524–535
Shine R, Downes SJ (1999) Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia 119:1–8
Shine R, Elphick M (2001) The effect of short-term weather fluctuations on temperatures inside lizard nests, and on the phenotypic traits of hatchling lizards. Biol J Linn Soc 72:555–565
Shine R, Harlow P (1993) Maternal thermoregulation influences offspring viability in a viviparous lizard. Oecologia 96:122–127
Shine R, Harlow PS (1996) Maternal manipulation of offspring phenotypes via nest-site selection in an oviparous lizard. Ecology 77:1808–1817
Shine R, Elphick MJ, Harlow PS (1997a) The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology 78:2559–2568
Shine R, Madsen TRL, Elphick MJ, Harlow PS (1997b) The influence of nest temperatures and maternal brooding on hatchling phenotypes of water pythons. Ecology 78:1713–1721
Shine R, Langkilde T, Wall M, Mason RT (2005) The fitness correlates of scalation asymmetry in garter snakes (Thamnophis sirtalis parietalis). Funct Ecol 19:306–314
Steffen W, Burbidge AA, Hughes L, Kitching R, Lindenmayer D, Musgrave W, Stafford Smith M, Werner PA (2009) Australia’s biodiversity and climate change: a strategic assessment of the vulnerability of Australia’s biodiversity to climate change, report to the Natural Resources Management Ministerial Council commissioned by the Australian Government, CSIRO Publishing, Canberra
Telemeco RS, Elphick M, Shine R (2009) Nesting lizards (Bassiana duperreyi) compensate partly, but not completely, for climate change. Ecology 90:17–22
Telemeco R, Radder RS, Baird TA, Shine R (2010) Thermal effects on reptile reproduction: Adaptation and phenotypic plasticity in a montane lizard. Biol J Linn Soc 100:642–655
Theurillat JP, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109
Waddington CH (1961) Genetic assimilation. Adv Genet 10:257–290
Wapstra E (2000) Maternal basking opportunity affects juvenile phenotype in a viviparous lizard. Funct Ecol 14:345–352
Wapstra E, Olsson M, Shine R, Edwards A, Swain R, Joss JMP (2004) Maternal basking behaviour determines offspring sex in a viviparous reptile. Biol Letters 271:S230–S232
Wapstra E, Uller T, Sinn DL, Olsson M, Mazurek K, Joss J, Shine R (2009) Climate effects on offspring sex ratio in a viviparous lizard. J Anim Ecol 78:84–90
Warner D, Shine R (2005) The adaptive significance of temperature-dependent sex determination: experimental tests with a short-lived lizard. Evolution 59:2209–2221
Warner DA, Shine R (2008) The adaptive significance of temperature-dependent sex determination in a reptile. Nature 451:566–568
Warner D, Woo K, Van Dyk D, Evans C, Shine R (2010) Egg incubation temperature affects male reproductive success but not display behaviors in lizards. Behav Ecol Sociobiol 64:803–813
Webb JK, Brown GP, Shine R (2001) Body size, locomotor speed and antipredator behaviour in a tropical snake (Tropidonophis mairii, Colubridae): the influence of incubation environments and genetic factors. Funct Ecol 15:561–568
Webb JK, Shine R, Christian KA (2006) The adaptive significance of reptilian viviparity in the tropics: testing the maternal manipulation hypothesis. Evolution 60:115–122
West-Eberhard MJ (2003) Development plasticity and evolution. Oxford University Press, Oxford
While GM, Uller T, Wapstra E (2009) Offspring performance and the adaptive benefits of prolonged pregnancy: experimental tests in a viviparous lizard. Funct Ecol 23:818–825
Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G (2008) Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biology 12:2621–2626
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dubey, S., Shine, R. Predicting the effects of climate change on reproductive fitness of an endangered montane lizard, Eulamprus leuraensis (Scincidae). Climatic Change 107, 531–547 (2011). https://doi.org/10.1007/s10584-010-9963-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10584-010-9963-x