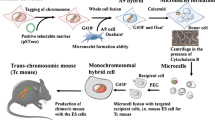
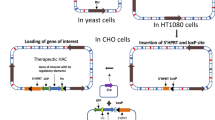
Abstract
Mammalian artificial chromosomes (MACs) are non-integrating, autonomously replicating natural chromosome-based vectors that may carry a vast amount of genetic material, which in turn enable potentially prolonged, safe, and regulated therapeutic transgene expression and render MACs as attractive genetic vectors for “gene replacement” or for controlling differentiation pathways in target cells. Satellite-DNA-based artificial chromosomes (SATACs) can be made by induced de novo chromosome formation in cells of different mammalian and plant species. These artificially generated accessory chromosomes are composed of predictable DNA sequences, and they contain defined genetic information. SATACs have already passed a number of obstacles crucial to their further development as gene therapy vectors, including large-scale purification, transfer of purified artificial chromosomes into different cells and embryos, generation of transgenic animals and germline transmission with purified SATACs, and the tissue-specific expression of a therapeutic gene from an artificial chromosome in the milk of transgenic animals. SATACs could be used in cell therapy protocols. For these methods, the most versatile target cell would be one that was pluripotent and self-renewing to address multiple disease target cell types, thus making multilineage stem cells, such as adult derived early progenitor cells and embryonic stem cells, as attractive universal host cells.

Similar content being viewed by others
Abbreviations
- ACEs:
-
Artificial chromosome expression system
- CHO:
-
Chinese hamster ovary
- EB:
-
Embryoid body
- EGFP:
-
Enhanced green fluorescent protein
- ESC:
-
Embryonic stem cell
- EPO:
-
Erythropoietin
- FACS:
-
Fluorescence-activated cell sorting
- HAC:
-
Human artificial chromosome
- hESC:
-
Human embryonic stem cell
- HGH:
-
Human growth hormone
- HPRT:
-
Hypoxanthine guanine phosphoribosyl transferase
- HSC:
-
Hematopoietic Stem Cell
- iPS:
-
Induced pluripotent stem
- MAbs:
-
Monoclonal antibodies
- MACs:
-
Mammalian artificial chromosomes
- MMCT:
-
Microcell-mediated chromosome transfer
- MRI:
-
Magnetic resonance imaging
- MSC:
-
Mesenchymal stem cell
- PET:
-
Positron emission tomography
- SATAC:
-
Satellite-DNA-based artificial chromosome
References
Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, Phelps DA, Remack JS, Yoon KJ, Gillespie S, Kim SU, Glackin CA, Potter PM, Danks MK (2006a) Development of a tumor-selective approach to treat metastatic cancer. PLoS One 1:e23–e23. doi:10.1371/journal.pone.0000023
Aboody KS, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, Przylecki W, Carroll R, Black PM, Perides G (2006b) Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro-Oncol 8:119–126. doi:10.1215/15228517-2005-012
Adhvaryu SG, Peters-Brown T, Livingston E, Qumsiyeh MB (1998) Familial supernumerary marker chromosome evolution through three generations. Prenat Diagn 18:178–181
Adriaansen J, Vervoordeldonk MJ, Vanderbyl S, de Jong G, Tak PP (2006) A novel approach for gene therapy: engraftment of fibroblasts containing the artificial chromosome expression system at the site of inflammation. J Gene Med 8:63–71. doi:10.1002/jgm.810
Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE (2002) Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng 8:541–550. doi:10.1089/107632702760240463
Ascenzioni F, Donini P, Lipps HJ (1997) Mammalian artificial chromosomes—vectors for somatic gene therapy. Cancer Lett 118:135–142
Atari M, Barajas M, Hernández-Alfaro F, Gil C, Fabregat M, Ferrés Padró E, Giner L, Casals N (2011) Isolation of pluripotent stem cells from human third molar dental pulp. Histol Histopathol 26:1057–1070
Atari M, Gil-Recio C, Fabregat M, García-Fernández D, Barajas M, Carrasco MA, Jung H-S, Alfaro FH, Casals N, Prosper F, Ferrés-Padró E, Giner L (2012) Dental pulp of the third molar: a new source of pluripotent-like stem cells. J Cell Sci 125:3343–3356. doi:10.1242/jcs.096537
Au HC, Mascarello JT, Scheffler IE (1999) Targeted integration of a dominant neo(R) marker into a 2- to 3-Mb human minichromosome and transfer between cells. Cytogenet Cell Genet 86:194–203
Auriche C, Donini P, Ascenzioni F (2001) Molecular and cytological analysis of a 5.5 Mb minichromosome. EMBO Rep 2:102–107. doi:10.1093/embo-reports/kve018
Auriche C, Carpani D, Conese M, Caci E, Zegarra-Moran O, Donini P, Ascenzioni F (2002) Functional human CFTR produced by a stable minichromosome. EMBO Rep 3:862–868. doi:10.1093/embo-reports/kvf174
Ayabe F, Katoh M, Inoue T, Kouprina N, Larionov V, Oshimura M (2005) A novel expression system for genomic DNA loci using a human artificial chromosome vector with transformation-associated recombination cloning. J Hum Genet 50:592–599. doi:10.1007/s10038-005-0300-6
Bagutti C, Wobus AM, Watt FM (1996) Differentiation of embryonal stem cells into keratinocytes: comparison of wild-type and beta 1 integrin-deficient cells. Dev Biol Dev Biol 179:184–196
Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MAS, Studer L (2003) Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol 21:1200–1207
Bar-Shir A, Liu G, Chan KWY, Oskolkov N, Song X, Yadav NN, Walczak P, McMahon MT, van Zijl PCM, Bulte JWM, Gilad AA (2014) Human protamine-1 as an MRI reporter gene based on chemical exchange. ACS Chem Biol 9:134–138. doi:10.1021/cb400617q
Basu J, Willard HF (2005) Artificial and engineered chromosomes: non-integrating vectors for gene therapy. Trends Mol Med 11:251–258. doi:10.1016/j.molmed.2005.03.006
Basu J, Willard HF (2006) Human artificial chromosomes: potential applications and clinical considerations. Pediatr Clin North Am 53:843–853. doi:10.1016/j.pcl.2006.08.013, viii
Basu J, Compitello G, Stromberg G, Willard HF, Van Bokkelen G (2005a) Efficient assembly of de novo human artificial chromosomes from large genomic loci. BMC Biotechnol 5:21–21. doi:10.1186/1472-6750-5-21
Basu J, Stromberg G, Compitello G, Willard HF, Van Bokkelen G (2005b) Rapid creation of BAC-based human artificial chromosome vectors by transposition with synthetic alpha-satellite arrays. Nucleic Acids Res 33:587–596. doi:10.1093/nar/gki207
Bautch VL, Stanford WL, Rapoport R, Russell S, Byrum RS, Futch TA (1996) Blood island formation in attached cultures of murine embryonic stem cells. Dev Dyn 205:1–12
Bernstein R, Dawson B, Griffiths J (1981) Human inherited marker chromosome 22 short-arm enlargement: investigation of rDNA gene multiplicity, Ag-band size, and acrocentric association. Hum Genet 58:135–139
Billon N, Jolicoeur C, Ying QL, Smith A, Raff M (2002) Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J Cell Sci 115:3657–3665
Blazso P, Sinko I, Katona RL (2011) Engineered chromosomes in transgenics. Methods Mol Biol Clifton NJ 738:161–181. doi:10.1007/978-1-61779-099-7_12
Blennow E, Bui TH, Kristoffersson U, Vujic M, Annerén G, Holmberg E, Nordenskjöld M (1994) Swedish survey on extra structurally abnormal chromosomes in 39 105 consecutive prenatal diagnoses: prevalence and characterization by fluorescence in situ hybridization. Prenat Diagn 14:1019–1028
Breman AM, Steiner CM, Slee RB, Grimes BR (2008) Input DNA ratio determines copy number of the 33 kb Factor IX gene on de novo human artificial chromosomes. Mol Ther J Am Soc Gene Ther 16:315–323. doi:10.1038/sj.mt.6300361
Bridger JM (2004) Mammalian artificial chromosomes: modern day feats of engineering—Isambard Kingdom Brunel style. Cytogenet Genome Res 107:5–8. doi:10.1159/000079563
Brøndum-Nielsen K, Mikkelsen M (1995) A 10-year survey, 1980–1990, of prenatally diagnosed small supernumerary marker chromosomes, identified by FISH analysis. Outcome and follow-up of 14 cases diagnosed in a series of 12,699 prenatal samples. Prenat Diagn 15:615–619
Brown WR, Mee PJ, Hong Shen M (2000) Artificial chromosomes: ideal vectors? Trends Biotechnol 18:218–223
Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD (1997) In vitro-generated neural precursors participate in mammalian brain development. Proc Natl Acad Sci U S A 94:14809–14814
Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD (1999) Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science 285:754–756
Bunnell BA, Izadpanah R, Ledebur HC Jr, Perez CF (2005) Development of mammalian artificial chromosomes for the treatment of genetic diseases: Sandhoff and Krabbe diseases. Expert Opin Biol Ther 5:195–206. doi:10.1517/14712598.5.2.195
Burt RK, Verda L, Kim DA, Oyama Y, Luo K, Link C (2004) Embryonic stem cells as an alternate marrow donor source: engraftment without graft-versus-host disease. J Exp Med 199:895–904
Cardinale S, Bergmann JH, Kelly D, Nakano M, Valdivia MM, Kimura H, Masumoto H, Larionov V, Earnshaw WC (2009) Hierarchical inactivation of a synthetic human kinetochore by a chromatin modifier. Mol Biol Cell 20:4194–4204. doi:10.1091/mbc.E09-06-0489
Carine K, Solus J, Waltzer E, Manch-Citron J, Hamkalo BA, Scheffler IE (1986) Chinese hamster cells with a minichromosome containing the centromere region of human chromosome 1. Somat Cell Mol Genet 12:479–491
Carl FP (2004) The ACE System: a versatile chromosome engineering technology with applications for gene-based cell therapy. Bioprocess J 61–68
Carotta S, Pilat S, Mairhofer A, Schmidt U, Dolznig H, Steinlein P, Beug H (2004) Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood 104:1873–1880
Cavazzana-Calvo M, Fischer A, Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, Radford I, Villeval J-L, Fraser CC (2003) A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 348:255–256. doi:10.1056/NEJM200301163480314
Chang AH, Stephan MT, Sadelain M (2006) Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotechnol 24:1017–1021
Choo KH, Saffery R, Wong LH, Irvine DV, Bateman MA, Griffiths B, Cutts SM, Cancilla MR, Cendron AC, Stafford AJ (2001) Construction of neocentromere-based human minichromosomes by telomere-associated chromosomal truncation. Proc Natl Acad Sci U S A 98:5705–5710. doi:10.1073/pnas.091468498
Christ M, Lusky M, Stoeckel F, Dreyer D, Dieterlé A, Michou AI, Pavirani A, Mehtali M (1997) Gene therapy with recombinant adenovirus vectors: evaluation of the host immune response. Immunol Lett 57:19–25
Co DO, Borowski AH, Leung JD, van der Kaa J, Hengst S, Platenburg GJ, Pieper FR, Perez CF, Jirik FR, Drayer JI (2000) Generation of transgenic mice and germline transmission of a mammalian artificial chromosome introduced into embryos by pronuclear microinjection. Chrom Res Int J Mol Supramol Evol Asp Chrom Biol 8:183–191
Colman A (2004) Making new beta cells from stem cells. Semin Cell Dev Biol 15:337–345
Conte RA, Kleyman SM, Laundon C, Verma RS (1997) Characterization of two extreme variants involving the short arm of chromosome 22: are they identical? Ann. Génétique 40:145–149
Cooke H (2001) Mammalian artificial chromosomes as vectors: progress and prospects. Cloning Stem Cells 3:243–249. doi:10.1089/15362300152725963
COOPER HL, HIRSCHHORN K (1962) Enlarged satellites as a familial chromosome marker. Am J Hum Genet 14:107–124
Coraux C, Hilmi C, Rouleau M, Spadafora A, Hinnrasky J, Ortonne JP, Dani C, Aberdam D (2003) Reconstituted skin from murine embryonic stem cells. Curr Biol 13:849–853
Couto LB (2004) Preclinical gene therapy studies for hemophilia using adeno-associated virus (AAV) vectors. Semin Thromb Hemost 30:161–171. doi:10.1055/s-2004-825630
Crolla JA, Howard P, Mitchell C, Long FL, Dennis NR (1997) A molecular and FISH approach to determining karyotype and phenotype correlations in six patients with supernumerary marker(22) chromosomes. Am J Med Genet 72:440–447
Csonka E (2011) De novo generation of satellite DNA-based artificial chromosomes by induced large-scale amplification. Methods Mol Biol Clifton NJ 738:111–125. doi:10.1007/978-1-61779-099-7_8
Csonka E, Cserpán I, Fodor K, Holló G, Katona R, Keresö J, Praznovszky T, Szakál B, Telenius A, deJong G, Udvardy A, Hadlaczky G (2000) Novel generation of human satellite DNA-based artificial chromosomes in mammalian cells. J Cell Sci 113(Pt 18):3207–3216
D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401
Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, Kim SU, Garcia E, Metz MZ, Najbauer J, Potter PM, Aboody KS (2007) Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res 67:22–25
De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A (2007) Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106
De Jong G, Telenius A, Vanderbyl S, Meitz A, Drayer J (2001) Efficient in-vitro transfer of a 60-Mb mammalian artificial chromosome into murine and hamster cells using cationic lipids and dendrimers. Chromosome Res Int J Mol Supramol Evol Asp Chromosome Biol 9:475–485
deJong G, Telenius AH, Telenius H, Perez CF, Drayer JI, Hadlaczky G (1999) Mammalian artificial chromosome pilot production facility: large-scale isolation of functional satellite DNA-based artificial chromosomes. Cytometry 35:129–133
do Pinto O, Kolterud A, Carlsson L (1998) Expression of the LIM-homeobox gene LH2 generates immortalized steel factor-dependent multipotent hematopoietic precursors. EMBO J 17:5744–5756
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87:27–45
Doherty AMO, Fisher EMC (2003) Microcell-mediated chromosome transfer (MMCT): small cells with huge potential. Mamm Genome Off J Int Mamm Genome Soc 14:583–592
Donovan PJ, de Miguel MP (2003) Turning germ cells into stem cells. Curr Opin Genet Dev 13:463–471
Duncan A, Hadlaczky G (2007) Chromosomal engineering. Curr Opin Biotechnol 18:420–424. doi:10.1016/j.copbio.2007.09.004
Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, Leavitt AD, Shattil SJ (2002) Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A 99:12819–12824
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Evans CH, Ghivizzani SC, Robbins PD (2008) Arthritis gene therapy’s first death. Arthritis Res Ther 10:110. doi:10.1186/ar2411
Fairchild PJ, Nolan KF, Cartland S, GraA a L, Waldmann H (2003) Stable lines of genetically modified dendritic cells from mouse embryonic stem cells. Transplantation 76:606–608
Farr CJ, Bayne RA, Kipling D, Mills W, Critcher R, Cooke HJ (1995) Generation of a human X-derived minichromosome using telomere-associated chromosome fragmentation. EMBO J 14:5444–5454
Fournier RE, Ruddle FH (1977) Microcell-mediated transfer of murine chromosomes into mouse, Chinese hamster, and human somatic cells. Proc Natl Acad Sci U S A 74:319–323
Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24:150–154
Friedrich U, Caprani M, Niebuhr E, Therkelsen AJ, Jørgensen AL (1996) Extreme variant of the short arm of chromosome 15. Hum Genet 97:710–713
Gilad AA, McMahon MT, Walczak P, Winnard PT, Raman V, van Laarhoven HWM, Skoglund CM, Bulte JWM, van Zijl PCM (2007) Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol 25:217–219. doi:10.1038/nbt1277
Goldman S (2005) Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol 23:862–871
Grimes BR, Monaco ZL (2005) Artificial and engineered chromosomes: developments and prospects for gene therapy. Chromosoma 114:230–241. doi:10.1007/s00412-005-0017-5
Grimes BR, Schindelhauer D, McGill NI, Ross A, Ebersole TA, Cooke HJ (2001) Stable gene expression from a mammalian artificial chromosome. EMBO Rep 2:910–914. doi:10.1093/embo-reports/kve187
Grimes BR, Rhoades AA, Willard HF (2002a) Alpha-satellite DNA and vector composition influence rates of human artificial chromosome formation. Mol Ther J Am Soc Gene Ther 5:798–805. doi:10.1006/mthe.2002.0612
Grimes BR, Warburton PE, Farr CJ (2002b) Chromosome engineering: prospects for gene therapy. Gene Ther 9:713–718. doi:10.1038/sj.gt.3301763
Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W, Hasenfuss G (2006) Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440:1199–1203
Guhr A, Kurtz A, Friedgen K, Peter L s (2006) Current state of human embryonic stem cell research: an overview of cell lines and their use in experimental work. Stem Cells 24:2187–2191
Gyöngyösi M, Blanco J, Marian T, Trón L, Petneházy O, Petrasi Z, Hemetsberger R, Rodriguez J, Font G, Pavo IJ, Kertész I, Balkay L, Pavo N, Posa A, Emri M, Galuska L, Kraitchman DL, Wojta J, Huber K, Glogar D (2008) Serial noninvasive in vivo positron emission tomographic tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging 1:94–103. doi:10.1161/CIRCIMAGING.108.797449
Hadlaczky G (2001) Satellite DNA-based artificial chromosomes for use in gene therapy. Curr Opin Mol Ther 3:125–132
Hadlaczky G, Praznovszky T, Cserpán I, Keresö J, Péterfy M, Kelemen I, Atalay E, Szeles A, Szelei J, Tubak V (1991) Centromere formation in mouse cells cotransformed with human DNA and a dominant marker gene. Proc Natl Acad Sci U S A 88:8106–8110
Hadlaczky G, Bujdosó G, Koltai E, Schwanitz G, Csonka E (2002) A natural equivalent to human satellite DNA-based artificial chromosome persists over 140 years, in a three-generation family. Eur J Hum Genet 10(Supplement):143–143
Hamaguchi-Tsuru E, Nobumoto A, Hirose N, Kataoka S, Fujikawa-Adachi K, Furuya M, Tominaga A (2004) Development and functional analysis of eosinophils from murine embryonic stem cells. Br J Haematol 124:819–827
Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N (2001) Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett 497:15–19
Harrington JJ, Van Bokkelen G, Mays RW, Gustashaw K, Willard HF (1997) Formation of de novo centromeres and construction of first-generation human artificial microchromosomes. Nat Genet 15:345–355. doi:10.1038/ng0497-345
Heller R, Brown KE, Burgtorf C, Brown WR (1996) Mini-chromosomes derived from the human Y chromosome by telomere directed chromosome breakage. Proc Natl Acad Sci U S A 93:7125–7130
Henning KA, Novotny EA, Compton ST, Guan XY, Liu PP, Ashlock MA (1999) Human artificial chromosomes generated by modification of a yeast artificial chromosome containing both human alpha satellite and single-copy DNA sequences. Proc Natl Acad Sci U S A 96:592–597
Hidaka K, Lee JK, Kim HS, Ihm CH, Iio A, Ogawa M, Nishikawa SI, Kodama I, Morisaki T (2003) Chamber-specific differentiation of Nkx2.5-positive cardiac precursor cells from murine embryonic stem cells. FASEB J 17:740–742
Hochedlinger K, Jaenisch R (2006) Nuclear reprogramming and pluripotency. Nature 441:1061–1067
Holló G, Keresö J, Praznovszky T, Cserpán I, Fodor K, Katona R, Csonka E, Fátyol K, Szeles A, Szalay AA, Hadlaczky G (1996) Evidence for a megareplicon covering megabases of centromeric chromosome segments. Chromosome Res Int J Mol Supramol Evol Asp Chromosome Biol 4:240–247
Houthuijzen JM, Daenen LGM, Roodhart JML, Voest EE (2012) The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer 106:1901–1906. doi:10.1038/bjc.2012.201
Howard-Peebles PN (1979) A familial bisatellited extra metacentric microchromosome in man. J Hered 70:347–348
Hung SC, Deng WP, Yang WK, Liu RS, Lee CC, Su TC, Lin RJ, Yang DM, Chang CW, Chen WH, Wei HJ, Gelovani JG (2005) Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res 11:7749–7756
Ikeno M, Grimes B, Okazaki T, Nakano M, Saitoh K, Hoshino H, McGill NI, Cooke H, Masumoto H (1998) Construction of YAC-based mammalian artificial chromosomes. Nat Biotechnol 16:431–439. doi:10.1038/nbt0598-431
Ikeno M, Inagaki H, Nagata K, Morita M, Ichinose H, Okazaki T (2002) Generation of human artificial chromosomes expressing naturally controlled guanosine triphosphate cyclohydrolase I gene. Genes Cells Devoted Mol Cell Mech 7:1021–1032
Ikeno M, Suzuki N, Hasegawa Y, Okazaki T (2009) Manipulating transgenes using a chromosome vector. Nucleic Acids Res 37:e44–e44. doi:10.1093/nar/gkp058
Inoue H, Nagata N, Kurokawa H, Yamanaka S (2014) iPS cells: a game changer for future medicine. EMBO J 33:409–417. doi:10.1002/embj.201387098
Irvine DV, Shaw ML, Choo KHA, Saffery R (2005) Engineering chromosomes for delivery of therapeutic genes. Trends Biotechnol 23:575–583. doi:10.1016/j.tibtech.2005.10.001
Ishida I, Kuroiwa Y, Tomizuka K, Shinohara T, Kazuki Y, Yoshida H, Ohguma A, Yamamoto T, Tanaka S, Oshimura M (2000) Manipulation of human minichromosomes to carry greater than megabase-sized chromosome inserts. Nat Biotechnol 18:1086–1090. doi:10.1038/80287
Jones EA, Tosh D, Wilson DI, Lindsay S, Forrester LM (2002) Hepatic differentiation of murine embryonic stem cells. Exp Cell Res 272:15–22
Kakeda M, Hiratsuka M, Nagata K, Kuroiwa Y, Kakitani M, Katoh M, Oshimura M, Tomizuka K (2005) Human artificial chromosome (HAC) vector provides long-term therapeutic transgene expression in normal human primary fibroblasts. Gene Ther 12:852–856
Kaname T, McGuigan A, Georghiou A, Yurov Y, Osoegawa K, De Jong PJ, Ioannou P, Huxley C (2005) Alphoid DNA from different chromosomes forms de novo minichromosomes with high efficiency. Chromosome Res Int J Mol Supramol Evol Asp Chromosome Biol 13:411–422. doi:10.1007/s10577-005-0979-4
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:557–563
Katoh M, Ayabe F, Norikane S, Okada T, Masumoto H, Horike S, Shirayoshi Y, Oshimura M (2004) Construction of a novel human artificial chromosome vector for gene delivery. Biochem Biophys Res Commun 321:280–290. doi:10.1016/j.bbrc.2004.06.145
Katona RL (2011) Dendrimer mediated transfer of engineered chromosomes. Methods Mol Biol Clifton NJ 738:151–160. doi:10.1007/978-1-61779-099-7_11
Katona RL, Sinkó I, Holló G, Szucs KS, Praznovszky T, Kereso J, Csonka E, Fodor K, Cserpán I, Szakál B, Blazsó P, Udvardy A, Hadlaczky G (2008) A combined artificial chromosome-stem cell therapy method in a model experiment aimed at the treatment of Krabbe’s disease in the Twitcher mouse. Cell Mol Life Sci CMLS 65:3830–3838. doi:10.1007/s00018-008-8442-2
Katona RL, Vanderbyl SL, Perez CF (2011) Mammalian artificial chromosomes and clinical applications for genetic modification of stem cells: an overview. Methods Mol Biol Clifton NJ 738:199–216. doi:10.1007/978-1-61779-099-7_14
Kawahara M, Inoue T, Ren X, Sogo T, Yamada H, Katoh M, Ueda H, Oshimura M, Nagamune T (2007) Antigen-mediated growth control of hybridoma cells via a human artificial chromosome. Biochim Biophys Acta 1770:206–212. doi:10.1016/j.bbagen.2006.10.014
Kazuki Y, Oshimura M (2011) Human artificial chromosomes for gene delivery and the development of animal models. Mol Ther J Am Soc Gene Ther 19:1591–1601. doi:10.1038/mt.2011.136
Kazuki Y, Hoshiya H, Kai Y, Abe S, Takiguchi M, Osaki M, Kawazoe S, Katoh M, Kanatsu-Shinohara M, Inoue K, Kajitani N, Yoshino T, Shirayoshi Y, Ogura A, Shinohara T, Barrett JC, Oshimura M (2008) Correction of a genetic defect in multipotent germline stem cells using a human artificial chromosome. Gene Ther 15:617–624. doi:10.1038/sj.gt.3303091
Kazuki Y, Hoshiya H, Takiguchi M, Abe S, Iida Y, Osaki M, Katoh M, Hiratsuka M, Shirayoshi Y, Hiramatsu K, Ueno E, Kajitani N, Yoshino T, Kazuki K, Ishihara C, Takehara S, Tsuji S, Ejima F, Toyoda A, Sakaki Y, Larionov V, Kouprina N, Oshimura M (2011) Refined human artificial chromosome vectors for gene therapy and animal transgenesis. Gene Ther 18:384–393. doi:10.1038/gt.2010.147
Kazuki K, Takehara S, Uno N, Imaoka N, Abe S, Takiguchi M, Hiramatsu K, Oshimura M, Kazuki Y (2013) Highly stable maintenance of a mouse artificial chromosome in human cells and mice. Biochem Biophys Res Commun 442:44–50. doi:10.1016/j.bbrc.2013.10.171
Kennard ML, Goosney DL, Monteith D, Roe S, Fischer D, Mott J (2009a) Auditioning of CHO host cell lines using the artificial chromosome expression (ACE) technology. Biotechnol Bioeng 104:526–539. doi:10.1002/bit.22407
Kennard ML, Goosney DL, Monteith D, Zhang L, Moffat M, Fischer D, Mott J (2009b) The generation of stable, high MAb expressing CHO cell lines based on the artificial chromosome expression (ACE) technology. Biotechnol Bioeng 104:540–553. doi:10.1002/bit.22406
Keresö J, Praznovszky T, Cserpán I, Fodor K, Katona R, Csonka E, Fátyol K, Holló G, Szeles A, Ross AR, Sumner AT, Szalay AA, Hadlaczky G (1996) De novo chromosome formations by large-scale amplification of the centromeric region of mouse chromosomes. Chromosome Res Int J Mol Supramol Evol Asp Chromosome Biol 4:226–239
Kim JH, Auerbach JM, Rodra-guez-Gamez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sanchez-Pernaute R, Bankiewicz K, McKay R (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418:50–56
Kim J-H, Kononenko A, Erliandri I, Kim T-A, Nakano M, Iida Y, Barrett JC, Oshimura M, Masumoto H, Earnshaw WC, Larionov V, Kouprina N (2011) Human artificial chromosome (HAC) vector with a conditional centromere for correction of genetic deficiencies in human cells. Proc Natl Acad Sci U S A 108:20048–20053. doi:10.1073/pnas.1114483108
Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R (2006) Human embryonic stem cell lines derived from single blastomeres. Nature 444:481–485
Klug MG, Soonpaa MH, Koh GY, Field LJ (1996) Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest 98:216–224
Koh BI, Kang Y (2012) The pro-metastatic role of bone marrow-derived cells: a focus on MSCs and regulatory T cells. EMBO Rep 13:412–422. doi:10.1038/embor.2012.41
Kolossov E, Fleischmann BK, Liu Q, Bloch W, Viatchenko-Karpinski S, Manzke O, Ji GJ, Bohlen H, Addicks K, Hescheler J (1998) Functional characteristics of ES cell-derived cardiac precursor cells identified by tissue-specific expression of the green fluorescent protein. J Cell Biol 143:2045–2056
Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL (2003) Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol 21:759–806
Kononenko AV, Lee NCO, Earnshaw WC, Kouprina N, Larionov V (2013) Re-engineering an alphoid(tetO)-HAC-based vector to enable high-throughput analyses of gene function. Nucleic Acids Res 41:e107. doi:10.1093/nar/gkt205
Kouprina N, Ebersole T, Koriabine M, Pak E, Rogozin IB, Katoh M, Oshimura M, Ogi K, Peredelchuk M, Solomon G, Brown W, Barrett JC, Larionov V (2003) Cloning of human centromeres by transformation-associated recombination in yeast and generation of functional human artificial chromosomes. Nucleic Acids Res 31:922–934
Kouprina N, Earnshaw WC, Masumoto H, Larionov V (2013) A new generation of human artificial chromosomes for functional genomics and gene therapy. Cell Mol Life Sci CMLS 70:1135–1148. doi:10.1007/s00018-012-1113-3
Kouprina N, Tomilin AN, Masumoto H, Earnshaw WC, Larionov V (2014) Human artificial chromosome-based gene delivery vectors for biomedicine and biotechnology. Expert Opin Drug Deliv 11:517–535. doi:10.1517/17425247.2014.882314
Kyba M, Perlingeiro RCR, Daley GQ (2002) HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109:29–37
Kyba M, Perlingeiro RCR, Hoover RR, Lu CW, Pierce J, Daley GQ (2003) Enhanced hematopoietic differentiation of embryonic stem cells conditionally expressing Stat5. Proc Natl Acad Sci U S A 100(Suppl 1):11904–11910
Larin Z, Mejía JE (2002) Advances in human artificial chromosome technology. Trends Genet TIG 18:313–319
Lewis M (2001) Human artificial chromosomes: emerging from concept to reality in biomedicine. Clin Genet 59:15–16
Li M, Pevny L, Lovell-Badge R, Smith A (1998) Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol 8:971–974
Li M, Liu G-H, Belmonte JCI (2012) Navigating the epigenetic landscape of pluripotent stem cells. Nat Rev Mol Cell Biol 13:524–535. doi:10.1038/nrm3393
Lieber JG, Webb S, Suratt BT, Young SK, Johnson GL, Keller GM, Worthen GS (2004) The in vitro production and characterization of neutrophils from embryonic stem cells. Blood 103:852–859
Lim HN, Farr CJ (2004) Chromosome-based vectors for mammalian cells: an overview. Methods Mol Biol Clifton NJ 240:167–186
Lin RY, Kubo A, Keller GM, Davies TF (2003) Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology 144:2644–2649
Lindenbaum M, Perkins E, Csonka E, Fleming E, Garcia L, Greene A, Gung L, Hadlaczky G, Lee E, Leung J, MacDonald N, Maxwell A, Mills K, Monteith D, Perez CF, Shellard J, Stewart S, Stodola T, Vandenborre D, Vanderbyl S, Ledebur HC (2004) A mammalian artificial chromosome engineering system (ACE System) applicable to biopharmaceutical protein production, transgenesis and gene-based cell therapy. Nucleic Acids Res 32:e172–e172. doi:10.1093/nar/gnh169
Lipps HJ, Jenke AC, Nehlsen K, Scinteie MF, Stehle IM, Bode J (2003) Chromosome-based vectors for gene therapy. Gene 304:23–33
Macnab S, Whitehouse A (2009) Progress and prospects: human artificial chromosomes. Gene Ther 16:1180–1188. doi:10.1038/gt.2009.102
Meaburn KJ, Parris CN, Bridger JM (2005) The manipulation of chromosomes by mankind: the uses of microcell-mediated chromosome transfer. Chromosoma 114:263–274. doi:10.1007/s00412-005-0014-8
Mejía JE, Willmott A, Levy E, Earnshaw WC, Larin Z (2001) Functional complementation of a genetic deficiency with human artificial chromosomes. Am J Hum Genet 69:315–326. doi:10.1086/321977
Messina G, Cossu G, Oshimura M, Hoshiya H, Kazuki Y, Abe S, Takiguchi M, Kajitani N, Watanabe Y, Yoshino T, Shirayoshi Y, Higaki K (2009) A highly stable and nonintegrated human artificial chromosome (HAC) containing the 2.4 Mb entire human dystrophin gene. Mol. Ther. J. Am. Soc. Gene Ther 17:309–317. doi:10.1038/mt.2008.253
Miller DG, Rutledge EA, Russell DW (2002) Chromosomal effects of adeno-associated virus vector integration. Nat Genet 30:147–148. doi:10.1038/ng824
Miller DG, Petek LM, Russell DW (2004) Adeno-associated virus vectors integrate at chromosome breakage sites. Nat Genet 36:767–773. doi:10.1038/ng1380
Mills W, Critcher R, Lee C, Farr CJ (1999) Generation of an approximately 2.4 Mb human X centromere-based minichromosome by targeted telomere-associated chromosome fragmentation in DT40. Hum Mol Genet 8:751–761
Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF (2002) Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol 92:288–296
Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, Morgan JP, Xiao YF (2003) Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg 125:361–369
Monaco ZL, Moralli D (2006) Progress in artificial chromosome technology. Biochem Soc Trans 34:324–327. doi:10.1042/BST20060324
Monteith DP, Leung JD, Borowski AH, Co DO, Praznovszky T, Jirik FR, Hadlaczky G, Perez CF (2004) Pronuclear microinjection of purified artificial chromosomes for generation of transgenic mice: pick-and-inject technique. Methods Mol Biol Clifton NJ 240:227–242
Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Hacein-Bey-Abina S, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Von Kalle C, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302:415–419. doi:10.1126/science.1088547
Mukerjee D, Burdette WJ (1966) A familial minute isochromosome. Am J Hum Genet 18:62–69
Müller M, Fleischmann BK, Selbert S, Ji GJ, Endl E, Middeler G, Müller OJ, Schlenke P, Frese S, Wobus AM, Hescheler J, Katus HA, Franz WM (2000) Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J 14:2540–2548
Nakano M, Cardinale S, Noskov VN, Gassmann R, Vagnarelli P, Kandels-Lewis S, Larionov V, Earnshaw WC, Masumoto H (2008) Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev Cell 14:507–522. doi:10.1016/j.devcel.2008.02.001
Nakashima H, Nakano M, Ohnishi R, Hiraoka Y, Kaneda Y, Sugino A, Masumoto H (2005) Assembly of additional heterochromatin distinct from centromere-kinetochore chromatin is required for de novo formation of human artificial chromosome. J Cell Sci 118:5885–5898. doi:10.1242/jcs.02702
Nakayama N, Lee J, Chiu L (2000) Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood 95:2275–2283
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348
Orkin SH, Hochedlinger K (2011) Chromatin connections to pluripotency and cellular reprogramming. Cell 145:835–850. doi:10.1016/j.cell.2011.05.019
Osakada F, Jin Z-B, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M (2009) In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci 122:3169–3179. doi:10.1242/jcs.050393
Oshimura M, Katoh M (2008) Transfer of human artificial chromosome vectors into stem cells. Reprod Biomed Online 16:57–69
Oshimura M, Tomizuka K, Shitara S, Kakeda M, Nagata K, Hiratsuka M, Sano A, Osawa K, Okazaki A, Katoh M, Kazuki Y (2008) Telomerase-mediated life-span extension of human primary fibroblasts by human artificial chromosome (HAC) vector. Biochem Biophys Res Commun 369:807–811. doi:10.1016/j.bbrc.2008.02.119
Otsuki A, Tahimic CGT, Tomimatsu N, Katoh M, Chen DJ, Kurimasa A, Oshimura M (2005) Construction of a novel expression system on a human artificial chromosome. Biochem Biophys Res Commun 329:1018–1025. doi:10.1016/j.bbrc.2005.02.079
Papp B, Plath K (2013) Epigenetics of reprogramming to induced pluripotency. Cell 152:1324–1343. doi:10.1016/j.cell.2013.02.043
Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong G, Hua G, Rosendahl A, Choi K (2004) A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development 131:2749–2762
Petrovic V, Stefanovic V (2009) Dental tissue—new source for stem cells. ScientificWorldJournal 9:1167–1177. doi:10.1100/tsw.2009.125
Praznovszky T, Keresö J, Tubak V, Cserpán I, Fátyol K, Hadlaczky G (1991) De novo chromosome formation in rodent cells. Proc Natl Acad Sci U S A 88:11042–11046
Raimondi E (2011) Naturally occurring minichromosome platforms in chromosome engineering: an overview. Methods Mol. Biol. Clifton NJ 738:41–56. doi:10.1007/978-1-61779-099-7_3
Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao G, Wilson JM, Batshaw ML (2003) Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80:148–158
Ren X, Katoh M, Hoshiya H, Kurimasa A, Inoue T, Ayabe F, Shibata K, Toguchida J, Oshimura M (2005) A novel human artificial chromosome vector provides effective cell lineage-specific transgene expression in human mesenchymal stem cells. Stem Cells Dayt Ohio 23:1608–1616. doi:10.1634/stemcells. 2005-0021
Ren X, Tahimic CGT, Katoh M, Kurimasa A, Inoue T, Oshimura M (2006) Human artificial chromosome vectors meet stem cells: new prospects for gene delivery. Stem Cell Rev 2:43–50. doi:10.1007/s12015-006-0008-9
Rocchi L, Braz C, Cattani S, Ramalho A, Christan S, Edlinger M, Ascenzioni F, Laner A, Kraner S, Amaral M, Schindelhauer D (2010) Escherichia coli-cloned CFTR loci relevant for human artificial chromosome therapy. Hum Gene Ther 21:1077–1092. doi:10.1089/hum.2009.225
Roodhart JML, Daenen LGM, Stigter ECA, Prins H-J, Gerrits J, Houthuijzen JM, Gerritsen MG, Schipper HS, Backer MJG, van Amersfoort M, Vermaat JSP, Moerer P, Ishihara K, Kalkhoven E, Beijnen JH, Derksen PWB, Medema RH, Martens AC, Brenkman AB, Voest EE (2011) Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell 20:370–383. doi:10.1016/j.ccr.2011.08.010
Sands VE (1969) Short arm enlargement in acrocentric chromosomes. Am J Hum Genet 21:293–304
Schmid M, Nanda I, Steinlein C, Epplen JT (1994) Amplification of (GACA)n simple repeats in an exceptional 14p + marker chromosome. Hum Genet 93:375–382
Schor SL, Johnson RT, Mullinger AM (1975) Perturbation of mammalian cell division. II. Studies on the isolation and characterization of human mini segregant cells. J Cell Sci 19:281–303
Scott CT, McCormick JB, DeRouen MC, Owen-Smith J (2011) Democracy derived? New trajectories in pluripotent stem cell research. Cell 145:820–826. doi:10.1016/j.cell.2011.05.032
Serafini M, Dylla SJ, Oki M, Heremans Y, Tolar J, Jiang Y, Buckley SM, Pelacho B, Burns TC, Frommer S, Rossi DJ, Bryder D, Panoskaltsis-Mortari A, O’Shaughnessy MJ, Nelson-Holte M, Fine GC, Weissman IL, Blazar BR, Verfaillie CM (2007) Hematopoietic reconstitution by multipotent adult progenitor cells: precursors to long-term hematopoietic stem cells. J Exp Med 204:129–139. doi:10.1084/jem.20061115
Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR, Gearhart JD (1998) Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A 95:13726–13731
Shen MH, Mee PJ, Nichols J, Yang J, Brook F, Gardner RL, Smith AG, Brown WR (2000) A structurally defined mini-chromosome vector for the mouse germ line. Curr Biol CB 10:31–34
Sofuni T, Tanabe K, Awa AA (1980) Chromosome heteromorphisms in the Japanese. II. Nucleolus organizer regions of variant chromosomes in D and G groups. Hum Genet 55:265–270
Stergianou K, Gould CP, Waters JJ, Hultén MA (1993) A DA/DAPI positive human 14p heteromorphism defined by fluorescence in-situ hybridisation using chromosome 15-specific probes D15Z1 (satellite III) and p-TRA-25 (alphoid). Hereditas 119:105–110
Stoffel M, Vallier L, Pedersen RA (2004) Navigating the pathway from embryonic stem cells to beta cells. Semin Cell Dev Biol 15:327–336
Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M (2004) Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96:1593–1603
Suda T, Katoh M, Hiratsuka M, Takiguchi M, Kazuki Y, Inoue T, Oshimura M (2006) Heat-regulated production and secretion of insulin from a human artificial chromosome vector. Biochem Biophys Res Commun 340:1053–1061. doi:10.1016/j.bbrc.2005.12.106
Suzuki N, Nishii K, Okazaki T, Ikeno M (2006) Human artificial chromosomes constructed using the bottom-up strategy are stably maintained in mitosis and efficiently transmissible to progeny mice. J Biol Chem 281:26615–26623. doi:10.1074/jbc.M603053200
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. doi:10.1016/j.cell.2007.11.019
THERKELSEN AJ (1964) Enlarged short arm of a small acrocentric chromosome in grandfather, mother and child, the latter with down’s syndrome. Cytogenetics 10:441–451
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Tóth A, Fodor K, Praznovszky T, Tubak V, Udvardy A, Hadlaczky G, Katona RL (2014) Novel method to load multiple genes onto a mammalian artificial chromosome. PLoS One 9:e85565. doi:10.1371/journal.pone.0085565
Trask B, van den Engh G, Gray JW (1989a) Inheritance of chromosome heteromorphisms analyzed by high-resolution bivariate flow karyotyping. Am J Hum Genet 45:753–760
Trask B, van den Engh G, Mayall B, Gray JW (1989b) Chromosome heteromorphism quantified by high-resolution bivariate flow karyotyping. Am J Hum Genet 45:739–752
Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D (2001) Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30:65–78
Tsai M, Wedemeyer J, Ganiatsas S, Tam SY, Zon LI, Galli SJ (2000) In vivo immunological function of mast cells derived from embryonic stem cells: an approach for the rapid analysis of even embryonic lethal mutations in adult mice in vivo. Proc Natl Acad Sci U S A 97:9186–9190
Tubak V, Hadlaczky G, Praznovszky T, Cserpán I, Keresö J, Péterfy M, Kelemen I, Atalay E, Szeles A, Szelei J (1991) Centromere formation in mouse cells cotransformed with human DNA and a dominant marker gene. Proc Natl Acad Sci U S A 88:8106–8110
Vanderbyl S, MacDonald GN, Sidhu S, Gung L, Telenius A, Perez C, Perkins E (2004) Transfer and stable transgene expression of a mammalian artificial chromosome into bone marrow-derived human mesenchymal stem cells. Stem Cells Dayt Ohio 22:324–333. doi:10.1634/stemcells.22-3-324
Vanderbyl SL, Sullenbarger B, White N, Perez CF, MacDonald GN, Stodola T, Bunnell BA, Ledebur HC, Lasky LC (2005) Transgene expression after stable transfer of a mammalian artificial chromosome into human hematopoietic cells. Exp Hematol 33:1470–1476. doi:10.1016/j.exphem.2005.08.008
Verma RS, Dosik H, Lubs HA (1977) Size variation polymorphisms of the short arm of human acrocentric chrosomes determined by R-banding by fluorescence using acridine orange (RFA). Hum Genet 38:231–234
Voet T, Vermeesch J, Carens A, Dürr J, Labaere C, Duhamel H, David G, Marynen P (2001) Efficient male and female germline transmission of a human chromosomal vector in mice. Genome Res 11:124–136
Warburton D, Atwood KC, Henderson AS (1976) Variation in the number of genes for rRNA among human acrocentric chromosomes: correlation with frequency of satellite association. Cytogenet Cell Genet 17:221–230
Wong LH, Saffery R, Choo KHA (2002) Construction of neocentromere-based human minichromosomes for gene delivery and centromere studies. Gene Ther 9:724–726. doi:10.1038/sj.gt.3301756
Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y (2002a) In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells 20:146–154
Yamada T, Yoshikawa M, Takaki M, Torihashi S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y (2002b) In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells 20:41–49
Yamada H, Kunisato A, Kawahara M, Tahimic CGT, Ren X, Ueda H, Nagamune T, Katoh M, Inoue T, Nishikawa M, Oshimura M (2006) Exogenous gene expression and growth regulation of hematopoietic cells via a novel human artificial chromosome. J Hum Genet 51:147–150. doi:10.1007/s10038-005-0334-9
Yamada H, Li YC, Nishikawa M, Oshimura M, Inoue T (2008) Introduction of a CD40L genomic fragment via a human artificial chromosome vector permits cell-type-specific gene expression and induces immunoglobulin secretion. J Hum Genet 53:447–453. doi:10.1007/s10038-008-0268-0
Yamanaka S (2012) Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10:678–684. doi:10.1016/j.stem.2012.05.005
Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S (2000) Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408:92–96
Yang Y, Min JY, Rana JS, Ke Q, Cai J, Chen Y, Morgan JP, Xiao YF (2002) VEGF enhances functional improvement of postinfarcted hearts by transplantation of ESC-differentiated cells. J Appl Physiol 93:1140–1151
Yoder FE, Bias WB, Borgaonkar DS, Bahr GF, Yoder II, Yoder OC, Golomb HM (1974) Cytogenetic and linkage studies of a familial 15pplus variant. Am J Hum Genet 26:535–548
Young HE, Duplaa C, Katz R, Thompson T, Hawkins KC, Boev AN, Henson NL, Heaton M, Sood R, Ashley D, Stout C, Morgan JH, Uchakin PN, Rimando M, Long GF, Thomas C, Yoon JI, Park JE, Hunt DJ, Walsh NM, Davis JC, Lightner JE, Hutchings AM, Murphy ML, Boswell E, McAbee JA, Gray BM, Piskurich J, Blake L, Collins JA, Moreau C, Hixson D, Bowyer FP, Black ACJ (2005) Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J Cell Mol Med 9:753–769
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KBS, Field LJ (2003) Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng 9:767–778
Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y (2006) Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol 80:267–74
Acknowledgments
I am greatly indebted to the late Gyula Hadlaczky for the discovery and development of SATACs, and also for facilitating me to work, learn, and progress scientifically under his kind supervision. Our work was supported by a Hungarian National Technology Program Grant: TECH_09_A1-DKMACTER (http://www.nih.gov.hu/english)
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editors: Natalay Kouprina and Vladimir Larionov.
Rights and permissions
About this article
Cite this article
Katona, R.L. De novo formed satellite DNA-based mammalian artificial chromosomes and their possible applications. Chromosome Res 23, 143–157 (2015). https://doi.org/10.1007/s10577-014-9458-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-014-9458-0