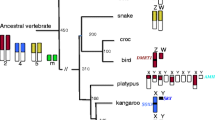
Abstract
Comparisons of the sex chromosome systems in birds and mammals are widening our view and deepening our understanding of vertebrate sex chromosome organization, function, and evolution. Birds have a very conserved ZW system of sex determination in which males have two copies of a large, gene-rich Z chromosome, and females have a single Z and a female-specific W chromosome. The avian ZW system is quite the reverse of the well-studied mammalian XY chromosome system, and evolved independently from different autosomal blocs. Despite the different gene content of mammal and bird sex chromosomes, there are many parallels. Genes on the bird Z and the mammal X have both undergone selection for male-advantage functions, and there has been amplification of male-advantage genes and accumulation of LINEs. The bird W and mammal Y have both undergone extensive degradation, but some birds retain early stages and some mammals terminal stages of the process, suggesting that the process is more advanced in mammals. Different sex-determining genes, DMRT1 and SRY, define the ZW and XY systems, but DMRT1 is involved in downstream events in mammals. Birds show strong cell autonomous specification of somatic sex differences in ZZ and ZW tissue, but there is growing evidence for direct X chromosome effects on sexual phenotype in mammals. Dosage compensation in birds appears to be phenotypically and molecularly quite different from X inactivation, being partial and gene-specific, but both systems use tools from the same molecular toolbox and there are some signs that galliform birds represent an early stage in the evolution of a coordinated system.



Similar content being viewed by others
Abbreviations
- ATP5A1:
-
ATP synthase, H+ transporting, mitochondrial F1 complex
- CHD:
-
Chromodomain helicase DNA binding protein
- DMRT1 (Dmrt1 in mice):
-
Doublesex and Mab3 related transcription factor
- DMY:
-
DM domain containing gene on the Y chromosome in medaka fish
- DMW:
-
DM domain containing gene on the W chromosome in Xenpous laevis
- FET-1:
-
Female-expressed transcript
- FEM1c:
-
Chicken homologue of C. elegans fem-1
- FISH:
-
Fluorescence in situ hybridization
- FOXL2:
-
Forkhead box L2
- GGA1:
-
Gallus gallus chromosome 1
- H4:
-
Histone H4
- HINTW:
-
Histidine triad nucleotide-binding protein 1
- LINEs:
-
Long interspersed nuclear elements
- MLH1:
-
Homologue of C. elegans protein MLH-1
- MHM:
-
Male hypermethylated region
- MY, MYA:
-
Million years (ago)
- RNAi:
-
Interfering RNA
- RSPO1:
-
Respondin 1
- SOX3, SOX9:
-
SRY-like HMG box-containing genes 3 and 9
- SRY:
-
Sex-determining region on the Y
- WNT4:
-
Wingless-type MMTV integration site, family member 4
References
Abdel-hameed F, Shoffner RN (1971) Intersexes and sex determination in chickens. Science 172:962–964
Adolfsson S, Ellegren H (2013) Lack of dosage compensation accompanies the arrested stage of sex chromosome evolution in ostriches. Mol Biol Evol 30:806–810
Aitken RJ, Graves JAM (2002) The future of sex. Nature 415:963
Arit D, Bensch S, Hansson B, Hasselquist D, Westerdahl H (2004) Observation of a ZZW female in a natural population: implications for avian sex determination. Proc Biol Sci 271(Suppl 4):S249–S251
Arnold AP, Chen X, Link JC, Itoh Y, Reue K (2013) Cell-autonomous sex determination outside of the gonad. Dev Dyn 242:371–379
Ayers KL, Davidson NM, Demiyah D et al (2013) RNA sequencing reveals sexually dimorphic gene expression before gonadal differentiation in chicken and allows comprehensive annotation of the W-chromosome. Genome Biol 14:R26
Bagheri-Fam S, Sinclair AH, Koopman P, Harley VR (2010) Conserved regulatory modules in the Sox9 testis-specific enhancer predict roles for SOX, TCF/LEF, Forkhead, DMRT, and GATA proteins in vertebrate sex determination. Int J Biochem Cell Biol 42:472–477
Bellott DW, Skaletsky H, Pytntikova T et al (2010) Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition. Nature 466:612–616
Berlin S, Ellegren H (2006) Fast accumulation of nonsynonymous mutations on the female-specific W chromosome in birds. J Mol Evol 62:66–72
Bisoni L, Batlle-Morera L, Bird AP, Suzuki M, Mcqueen HA (2005) Female-specific hyperacetylation of histone H4 in the chicken Z chromosome. Chrom Res 13:205–214
Blagoveshchenskii I, Sazanova AL, Stekol’nikova VA et al (2011) Investigation of pseudoautosomal and bordering regions in avian Z and W chromosomes with the use of large insert genomic BAC clones. Genetika 47:312–319
Bull JJ (1983) Evolution of sex determining mechanisms. The Benjamin/Cummings Publishing Company, California
Cai Q, Qian X, Lang Y et al (2013) Genome sequence of ground tit Pseudopodoces humilis and its adaptation to high altitude. Genome Biol 14(3):R29
Ceplitis H, Ellegren H (2004) Adaptive molecular evolution of HINTW, a female-specific gene in birds. Mol Biol Evol 21:249–254
Charlesworth B (1991) The evolution of sex chromosomes. Science 251:1030–1033
Chen N, Bellott DW, Page DC, Clark AG (2012) Identification of avian W-linked contigs by short-read sequencing. BMC Genom 13:183
Chue J, Smith CA (2011) Sex determination and sexual differentiation in the avian model. FEBS J 278:1027–1034
Collins KE, Jordan BJ, Mclendon BL, Navara KJ, Beckstead RB, Wilson JL (2013) No evidence of temperature-dependent sex determination or sex-biased embryo mortality in the chicken. Poult Sci 92:3096–3102
Cutting AD, Bannister SC, Doran TJ, Sinclair AH, Tizard MV, Smith CA (2012) The potential role of microRNAs in regulating gonadal sex differentiation in the chicken embryo. Chrom Res 20:201–213
Cutting A, Chue J, Smith CA (2013) Just how conserved is vertebrate sex determination? Dev Dyn 242:380–387
Disteche CM (2012) Dosage compensation of the sex chromosomes. Annu Rev Genet 46:537–560
DuRant SE, Hopkins WA, Hepp GR, Walters JR (2013) Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biol Rev Camb Philos Soc 88:499–509
Ellegren H (1996) First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc Biol Sci 263:1635–1641
Ellegren H (2003) Levels of polymorphism on the sex-limited chromosome: a clue to Y from W? Bioessays 25:163–167
Ellegren H, Hultin-Rosenberg L, Brunström B, Dencker L, Kultima K, Scholz B (2007) Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol 5:40
Ellegren H (2009) Genomic evidence for a large-Z effect. Proc Biol Sci 276:361–366
Ellegren H (2010) Evolutionary stasis: the stable chromosomes of birds. Trends Ecol Evol 25:283–291
Ellegren H, Smeds L, Burri R et al (2012) The genomic landscape of species divergence in Ficedula flycatchers. Nature 491(7426):756–760
Ezaz T, Stiglec R, Veyrunes F, Graves JAM (2006) Relationships between vertebrate ZW and XY sex chromosome systems. Curr Biol 16:R736–R743
Ezaz T, Azad B, O’Meally D, Young MJ et al (2013) Sequence and gene content of a large fragment of a lizard sex chromosome and evaluation of candidate sex differentiating gene R-spondin 1. BMC Genomics 14:899
Goerlich-Jansson VC, Muller MS, Groothuis TG (2013) Manipulation of primary sex ratio in birds: lessons from the homing pigeon (Columba livia domestica). Integr Comp Biol 53:902–912
Göth A, Booth DT (2005) Temperature-dependent sex ratio in a bird. Biol Lett 1:31–33
Graves JAM (1995) The origin and function of the mammalian Y chromosome and Y-borne genes—an evolving understanding. Bioessays 17:311–320
Graves JAM (2003) Sex and death in birds: a model of dosage compensation that predicts lethality of sex chromosome aneuploids. Cytogenet Genom Res 101:278–282
Graves JAM (2006) Sex chromosome specialization and degeneration in mammals. Cell 124:901–914
Graves JAM (2008) Weird animal genomes and the evolution of sex and sex chromosomes. Annu Rev Genet 42:565–586
Graves JAM, Peichel CL (2010) Are homologies in vertebrate sex determination due to shared ancestry or limited options? Genome Biol 11:205pp
Graves JAM (2013) How to evolve new vertebrate sex determining genes. Dev Dyn 242:354–359
Graves JAM, Ddisteche CM, Toder R (1998) Gene dosage in the evolution and function of mammalian sex chromosomes. Cytogenet Cell Genet 80:94–103
Griffen DK (2012) Is the Y chromosome disappearing?—both sides of the argument. Chrom Res 20:35–45
Groenen MA, Cheng HH, Bumstead N et al (2000) A consensus linkage map of the chicken genome. Genome Res 10:137–147
Guioli S, Lovell-Badge R, Turner JM (2012) Error-prone ZW pairing and no evidence for meiotic sex chromosome inactivation in the chicken germ line. PLoS Genet 8(3):e1002560
International Chicken Genome Sequencing Consortium (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432:695–716
Itoh Y, Melamed E, Yang X et al (2007) Dosage compensation is less effective in birds than in mammals. J Biol 6:2
Itoh Y, Kampf K, Ap A (2009) Disruption of FEM1C-W gene in zebra finch: evolutionary insights on avian ZW genes. Chromosoma 118:323–334
Itoh Y, Replogle K, Kim YH, Wade J, Clayton DF, Arnold AP (2010) Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res 20:512–518
Itoh Y, Kampf K, Arnold AP (2011) Possible differences in the two Z chromosomes in male chickens and evolution of MHM sequences in Galliformes. Chromosoma 120:587–598
Janes DE, Ezaz T, Graves JAM, Edwards SV (2009) Recombination and nucleotide diversity in the sex chromosomal pseudoautosomal region of the Emu, Dromaius novaehollandiae. J Hered 100:125–136
Julien P, Brawand D, Soumillon M et al (2012) Mechanisms and evolutionary patterns of mammalian and avian dosage compensation. PLoS Biol 10(5):e1001328
Kawai A, Ishijima J, Nishida C (2008) The ZW sex chromosomes of Gekko hokouensis (Gekkonidae, Squamata) represent highly conserved homology with those of avian species. Chromosoma 118:43–51
Küpper C, Augustin J, Edwards S et al (2012) Triploid plover female provides support for a role of the W chromosome in avian sex determination. Biol Lett 8:787–789
Kuroda Y, Arai N, Arita M (2001) Absence of Z-chromosome inactivation for five genes in male chickens. Chrom Res 9:457–468
Li Y, Zhang L, Zhang D, Zhang X, Lu X (2010) Faster evolution of Z-linked duplicate genes in chicken. J Genet Genom 37:695–702
Lin M, Thorne MH, Martin IC, Sheldon BL, Jones RC (1995) Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW). Reprod Fertil Dev 7:1185–1197
Livernois AM, Graves JA, Waters PD (2012) The origin and evolution of vertebrate sex chromosomes and dosage compensation. Heredity (Edinb) 108:50–58
Livernois AM, Al Nadaf S, Deakin JE, Graves JAM, Waters PD (2013) Independent evolution of transcriptional inactivation on sex chromosomes in birds and mammals. PLoS Genet 9:e1003635
Mank JE, Axelsson E, Ellegren H (2007) Fast-X on the Z: rapid evolution of sex-linked genes in birds. Genome Res 17:618–624
Mank JE, Ellegren H (2007) Parallel divergence and degradation of the avian W sex chromosome. Trends Ecol Evol 22:389–391
Mank JE, Ellegren H (2009a) Sex-linkage of sexually antagonistic genes is predicted by female, but not male, effects in birds. Evolution 63:1464–1472
Mank JE, Ellegren H (2009b) All dosage compensation is local: gene-by-gene regulation of sex-biased expression on the chicken Z chromosome. Heredity 102:312–320
Mank JE, Nam K, Ellegren H (2010) Faster-Z evolution is predominantly due to genetic drift. Mol Biol Evol 27:661–670
Matson CK, Zarkower D (2012) Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet 13:163–174
Matsubara K, Tarui H, Toriba M, Yamada K, Nishida-Umehara C, Agata K, Matsuda Y (2006) Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc Natl Acad Sci U S A 103:18190–18195
McQueen HA, McBride D, Miele G, Bird AP, Clinton M (2001) Dosage compensation in birds. Curr Biol 11:253–257
Moghadam HK, Pointer MA, Wright AE, Berlin S, Mank JE (2012) W chromosome expression responds to female-specific selection. Proc Natl Acad Sci U S A 109:8207–8211
Nanda I, Schlegelmilch K, Haaf T, Schartl M, Schmid M (2008) Synteny conservation of the Z chromosome in 14 avian species (11 families) supports a role for Z dosage in avian sex determination. Cytogenet Genom Res 122:150–156
Nanda I, Shan Z, Schartl M et al (1999) 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat Genet 21:258–259
Nanda I, Benisch P, Fetting D, Haaf T, Schmid M (2011) Synteny conservation of chicken macrochromosomes 1–10 in different avian lineages revealed by cross-species chromosome painting. Cytogenet Genom Res 132:165–181
Naurin S, Hansson B, Bensch S, Hasselquist D (2010) Why does dosage compensation differ between XY and ZW taxa? Trends Genet 26:15–20
Naurin S, Hasselquist D, Bensch S, Hansson B (2012) Sex-biased gene expression on the avian Z chromosome: highly expressed genes show higher male-biased expression. PLoS One 7:e46854
Nishida-Umehara C, Tsuda Y, Ishijima J et al (2007) The molecular basis of chromosome orthologies and sex chromosomal differentiation in palaeognathous birds. Chrom Res 15:721–734
Nishida-Umehara C, Fujiwara A, Ogawa A, Mizuno S, Abe S, Yoshida MC (1999) Differentiation of Z and W chromosomes revealed by replication banding and FISH mapping of sex-chromosome-linked DNA markers in the cassowary (Aves, Ratitae). Chrom Res 7:635–640
Ogawa A, Murata K, Mizuno S (1998) The location of Z- and W-linked marker genes and sequence on the homomorphic sex chromosomes of the ostrich and the emu. Proc Natl Acad Sci U S A 95:4415–4418
Ohno S (1967) Sex chromosomes and sex-linked genes. Springer, New York
O’Meally D, Ezaz T, Georges A, Sarre SD, Graves JAM (2012) Are some chromosomes particularly good at sex? Insights from amniotes. Chrom Res 20:7–19
Pala I, Naurin S, Stervander M, Hasselquist D, Bensch S, Hansson B (2012) Evidence of a neo-sex chromosome in birds. Heredity (Edinb) 108:264–272
Pessia E, Engelstädter J, Marais GA (2013) The evolution of X chromosome inactivation in mammals: the demise of Ohno’s hypothesis? Cell Mol Life Sci. XXXX
Pigozzi MI (2011) Diverse stages of sex-chromosome differentiation in tinamid birds: evidence from crossover analysis in Eudromia elegans and Crypturellus tataupa. Genetica 139:771–777
Pokorná M, Giovannotti M, Kratochvíl L et al (2011) Strong conservation of the bird Z chromosome in reptilian genomes is revealed by comparative painting despite 275 million years divergence. Chromosoma 120:455–468
Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D (1999) Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol 215:208–220
Roeszler KN, Itman C, Sinclair AH, Smith CA (2012) The long non-coding RNA, MHM, plays a role in chicken embryonic development, including gonadogenesis. Dev Biol 366:317–326
Schoenmakers S, Wassenaar E, Hoogerbrugge JW, Laven JS, Grootegoed JA, Baarends WM (2009) Female meiotic sex chromosome inactivation in chicken. PLoS Genet 5:e1000466
Sekido R, Lovell-Badge R (2013) Genetic control of testis development. Sex Dev 7:21–32
Sharman GB (1969) The sex chromosomes of intersexual marsupials. J Reprod Fertil 19:388–389
Shetty S, Griffin DK, Graves JAM (1999) Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chrom Res 7:289–295
Shetty S, Kirby P, Zarkower D, Graves JAM (2002) DMRT1 in a ratite bird: evidence for a role in sex determination and discovery of a putative regulatory element. Cytogenet Genom Res 99:245–251
Smith CA, McClive PJ, Western PS, Reed KR, Sinclair AH (1999) Conservation of a sex-determining gene. Nature 402:601–602
Smith CA (2007) Sex determination in birds: HINTs from the W sex chromosome? Sex Dev 1:279–285
Smith CA, Roeszler KN, Ohnesorg T et al (2009a) The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 461:267–271
Smith CA, Roeszler KN, Sinclair AH (2009b) Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. Int J Dev Biol 53:59–67
Solari AJ (1992) Equalization of Z and W axes in chicken and quail oocytes. Cytogenet Cell Genet 59:52–56
Solari AJ (1994) Sex chromosomes and sex determination in vertebrates. CRC Press, Boca Raton
Suh A, Kriegs JO, Brosius J, Schmitz J (2011) Retroposon insertions and the chronology of avian sex chromosome evolution. Mol Biol Evol 28:2993–2997
Teranishi M, Shimada Y, Hori et al (2001) Transcripts of the MHM region on the chicken Z chromosome accumulate as non-coding RNA in the nucleus of female cells adjacent to the DMRT1 locus. Chrom Res 9:147–165
Tsuda Y, Nishida-Umehara C, Ishijima J, Yamada K, Matsuda Y (2007) Comparison of the Z and W sex chromosomal architectures in elegant crested tinamou (Eudromia elegans) and ostrich (Struthio camelus) and the process of sex chromosome differentiation in palaeognathous birds. Chromosoma 116:159–173
Uebbing S, Kunstner A, Makinen H, Ellegren H (2013) Transcriptome sequencing reveals the character of incomplete dosage compensation across multiple tissues in flycatchers. Genom Biol Evol 5:1555–1566
Veyrunes F, Waters PD, Miethke P et al (2008) Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes. Genome Res 18:965–973
Vicoso B, Kaiser VB, Bachtrog D (2013) Sex-biased gene expression at homomorphic sex chromosomes in emus and its implication for sex chromosome evolution. Proc Natl Acad Sci U S A 110:6453–6458
Warren W, Clayton DF, Ellegren H, Arnold AP et al (2010) The genome of a songbird. Nature 464:757–762
Wolf JBW, Bryk J (2011) General lack of global dosage compensation in ZZ/ZW systems? Broadening the perspective with RNA-seq. BMC Genomics 12:91
Zarkower D (2013) DMRT genes in vertebrate gametogenesis. Curr Top Dev Biol 102:327–356
Zhang SO, Mathur S, Hattem G, Tassy O, Pourquié O (2010) Sex-dimorphic gene expression and ineffective dosage compensation of Z-linked genes in gastrulating chicken embryos. BMC Genomics 11:13
Zhao D, McBride D, Nandi S et al (2010) Somatic sex identity is cell autonomous in the chicken. Nature 464:237–242
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Darren K. Griffin and Beth A. Sullivan.
Rights and permissions
About this article
Cite this article
Graves, J.A.M. Avian sex, sex chromosomes, and dosage compensation in the age of genomics. Chromosome Res 22, 45–57 (2014). https://doi.org/10.1007/s10577-014-9409-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-014-9409-9