Abstract
According to the “immortal” DNA strand hypothesis (Cairns Nature 255:197–200, 1975), stem cells would keep their template strands in order to prevent the accumulation of mutations, which could occur during DNA replication. Despite the growing number of studies that attempt to test this hypothesis, the conclusions remain highly controversial. In the base of this controversy lie the current limitations of available methodology to selectively and faithfully track the fate of template DNA strands throughout and upon cell division. Here, we developed a method that allows the unequivocal tracking of single chromatids containing template DNA strands in Drosophila S2 cells in culture. This method consists in the induction of mitosis with unreplicated genomes (MUGs) in which cells are allowed to enter mitosis without prior DNA replication. This is achieved by RNAi-mediated knockdown of Double parked, a conserved protein required for the initiation of DNA replication and post-replication checkpoint response. The advantages of this system when compared with MUGs generated in mammalian cells is the preservation of chromatid morphology, the ease of loss-of-function studies and the possibility of in vivo applications. Altogether, this approach allows for the readily visualization and tracking of template DNA strands by simply monitoring cells stably expressing GFP-fusions with either Histone H2B or the centromeric Histone variant CID/CENP-A by time-lapse fluorescence microscopy. This might be useful for the dissection of the molecular mechanism behind asymmetric DNA strand segregation.
Similar content being viewed by others
Introduction
Correct segregation of genetic material into daughter cells during mitosis depends on previously completed replication of DNA during S phase. In 1975, John Cairns (Cairns 1975), based on previous reports of non-random segregation of sister chromatids in mammalian cells (Lark et al. 1966), proposed the “immortal strand” hypothesis according to which stem cells would retain template DNA strands, whereas newly synthesized DNA replicas would segregate into differentiating daughter cells. By keeping template strands, stem cells would avoid the accumulation of mutations that could occur during DNA replication. This hypothesis implies a biased/asymmetric distribution of sister chromatids, but the underlying mechanism remains unknown (Tajbakhsh and Gonzalez 2009). Some possibilities include differences in gene expression, chromatin structure, chromatin modifications between the strands or asymmetry of mitotic spindle components such as centrosomes (Lew et al. 2008, Tajbakhsh and Gonzalez 2009, Charville and Rando 2011).
Support for and against asymmetric segregation of all or just a few DNA strands associated with specific chromosomes can be found in the literature in the most diversified systems, from yeast to man (Tajbakhsh 2008, Rocheteau et al. 2012, Escobar et al. 2011, Schepers et al. 2011, Armakolas and Klar 2006, Conboy et al. 2007, Armakolas et al. 2010), making this a highly controversial topic, mostly due to current limitations in the molecular identification of truly stem cell populations and to selectively label and track template DNA strands throughout and upon cell division. The latter traditionally involves the administration of labelled nucleotides (e.g., 5-bromo-deoxyuridine or H3-thymidine) that will mark older or newer DNA strands, depending on the protocol used, but which cannot reliably distinguish stem cells from differentiated cells with a slow cell cycle. Therefore, finding a good model system that would allow not only the monitorization of chromatids containing template DNA strands at high spatial and temporal resolution, but also the dissection of the molecular mechanism that could account for biased/asymmetric strand segregation during cell division will be detrimental to complement existing methodology.
Although correct and complete DNA replication is required to allow cells to proceed with the cell cycle, mammalian cells treated with hydroxyurea (HU) and caffeine can enter mitosis without previously replicating their genomes (Brinkley et al. 1988). Hydroxyurea blocks DNA replication, whereas caffeine allows the override of a post-replication checkpoint. This phenomenon known as mitosis with unreplicated genomes (MUGs) has been used in a number of different studies to demonstrate that kinetochores, which detach from chromatin in mammalian MUGs, are sufficient to interact with and be segregated autonomously by the mitotic spindle (Brinkley et al. 1988, Wise and Brinkley 1997, O'Connell et al. 2008, O'Connell et al. 2009, Johnson and Wise 2010). An important corollarium from these experiments is that MUGs can satisfy the spindle assembly checkpoint (SAC) and proceed with anaphase (Brinkley et al. 1988, O'Connell et al. 2008), thereby allowing the subsequent tracking and fate determination of the segregated DNA.
Drosophila Double parked (Dup) is a conserved origin of replication protein that is essential for the initiation of DNA replication in S phase and is involved in a post-replication checkpoint, thereby preventing cells to enter mitosis without completing DNA replication (Whittaker et al. 2000). We reasoned that Dup knockdown by RNAi would drive cells into mitosis without previous DNA replication, which would represent an efficient way to generate and selectively track single chromatids containing template DNA strands. As proof-of-principle, we tested this idea in Drosophila S2 cells in culture, in which it is possible to monitor chromosome and mitotic spindle behaviour throughout cell division by stably expressing GFP (or any other fluorescent protein) fusion proteins with core chromosomal and spindle apparatus components. Due to their simple cytology that favours high-resolution imaging studies, the ease of loss-of-function studies by RNAi (Moutinho-Pereira et al. 2010), together with the capacity to induce polarity and asymmetric division in Drosophila S2 cells (Johnston et al. 2009), this system may be potentially suited to complement existing methods for the dissection of the molecular mechanism behind asymmetric DNA strand segregation.
Materials and methods
Cell lines
Drosophila S2 cells stably expressing either GFP-H2B Histone and mCherry-α-tubulin (Maiato and Lince-Faria 2010) or GFP-α-tubulin and CID-mCherry (Moutinho-Pereira et al. 2010), were grown at 25 °C in Schneider’s medium (Invitrogen).
RNA interference
Dup dsRNA was obtained following the protocol previously described in (Maiato et al. 2003). For synthetizing Dup dsRNA from Drosophila S2 cells genomic DNA, we used the primers: forward TAATACGACTCACTATAGGGGTCATAACGTGTGGATTCATGG and reverse TAATACGACTCACTATAGGGCAAGACTCCCACAAAAATACCG. For RNAi of Dup 106 S2 cells were seeded in six well plates and incubated with 1 ml of Schneider´s medium without FBS and containing 10 μg/ml of Dup dsRNA for 1 h (Moutinho-Pereira et al. 2010). After incubation, 2 ml of Schneider´s medium with FBS were added and cells were kept for 96 h at 25 °C.
Imaging
Dup-depleted S2 cells stably expressing GFP-H2B Histone /mCherry-α-tubulin or GFP-α-tubulin/CID-mCherry were plated on 0.25 mg/ml concanavalin A-coated glass-bottom dishes (MatTek) and multipoint time-lapse images of living cells were obtained using a Nikon TE2000U inverted microscope equipped with a Yokogawa CSU-X1 spinning-disc confocal head, two laser lines (488 and 561 nm) and a motorized stage (Marzhauser). Images were collected as a multi (9) 0.5 μm separated z-planes with a time interval of 2 min and detected by an iXonEM + Electron Multiplying CCD camera (Andor). An effective pixel size of 0.076 μm was achieved using ×100 1.4 NA plan-Apochromatic DIC objective and two additional lenses: an ×1.5 optivar and a ×1.41 plan-apochromatic imaging lens before the CCD. The system was controlled by NIS-elements software (Nikon, Japan) and videos were processed and analysed with ImageJ.
Results
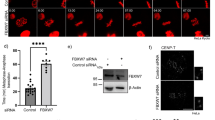
With the aim of establishing a tractable cell culture system undergoing MUGs in Drosophila we performed Dup Histone knockdown by RNAi in S2 cells stably expressing GFP-H2B Histone /mCherry-α-tubulin or GFP-α-tubulin/CID-mCherry. To confirm that Dup-depleted cells entered mitosis without DNA replication we performed live cell imaging using a spinning-disc confocal microscope. As opposed to mammalian cell MUGs in which unreplicated DNA remains decondensed, Dup-depleted S2 cells preserved chromatid morphology (Fig. 1b–e, Movies S2–5), which we used as read-out to validate our experimental strategy. Typically, control S2 cells stably expressing GFP-H2B Histone /mCherry-α-tubulin or GFP-α-tubulin/CID-mCherry established a bipolar spindle with bioriented chromosomes aligned at the metaphase plate and mitosis was completed in 32 ± 9 min (mean ± SD; n = 11 cells) (Fig. 1a, Movie S1 and Fig. 2a, Movie S6). In agreement with previous studies in Drosophila embryos (Parry et al. 2003), Dup-depleted S2 cells entered mitosis with single chromatids (Fig. 1b–e, Movies S2-5). This allowed the unequivocal tracking of chromatids containing template DNA strands. Accordingly, single chromatids upon Dup depletion remained scattered up to several hours (Table 1), likely due to the establishment of unstable kinetochore attachments with spindle microtubule plus ends as result of reduced/absent centromeric tension (King and Nicklas 2000, Pinsky and Biggins 2005). Due to these unstable attachments a highly variable number of chromosomes/kinetochores remained close to the poles throughout mitosis (Fig. 1b–e, Movies S2–5, Fig. 2b, c and Movies S7–8). Interestingly, three out of four Dup-depleted cells (Table 1) showed a clear asymmetric distribution of chromosomes/kinetochores between the two poles immediately upon nuclear envelope breakdown and establishment of initial kinetochore-microtubule attachments (Fig. 2b–c, Movies S7–8). This correlated with a much higher microtubule organizing activity from one of the centrosomes, and spindle bipolarity was achieved by means of centrosome-independent mechanisms (Maiato et al. 2004). Importantly, all recorded Drosophila S2 cells undergoing MUGs eventually exited mitosis (Table 1), as determined by spindle and cell elongation together with chromatin decondensation (Fig. 1b–e, Movies S2–5). This is consistent with previous studies in mammalian cells undergoing MUGs, which were shown to satisfy the SAC after a mitotic delay (Brinkley et al. 1988, O'Connell et al. 2008). Curiously, mammalian cells undergoing MUGs do not seem to elongate the spindle during anaphase B (Johnson and Wise 2010), which was not the case in Drosophila S2 cells (Fig. 1e, Movie S5 and Table 1).
Dup-depleted cells enter mitosis with unreplicated genomes (MUGs) with single, condensed chromatids. Live cell imaging of control and Dup-depleted Drosophila S2 cells stably expressing GFP-H2B Histone (shown in green) and mCherry-α-tubulin (shown in red). a Control cells containing replicated chromosomes aligned during metaphase and segregated evenly during anaphase. b–e Dup-depleted S2 cells enter MUGs with single, condensed chromatids (arrows) which remain scattered within the mitotic spindle. Spindle elongation and DNA decondensation can be observed at the end of MUGs. Scale bar 5 μm. Time is in h:min
Dup-depleted cells undergoing MUGs contain single kinetochores improperly attached to the mitotic spindle. Live cell imaging of control and Dup-depleted Drosophila S2 cells stably expressing GFP-α-tubulin (shown in red) and CID-mCherry (shown in green). a Control cells contain paired, bioriented kinetochores with amphitelic attachments (boxed, enlarged). b–c Single, unpaired kinetochores of Dup-depleted cells undergoing MUGs. c Kinetochores during MUGs are either merotelic (left box) or monotelic (right box) attached (arrowheads on microtubules). b Arrow is pointing to the single pole excluded from the mitotic spindle showing lower microtubule organization capacity. Scale bar, 5 μm. Time is in h:min
Contrary to control Drosophila S2 cells in which chromosomes segregated evenly during anaphase (Fig. 1a, Movie S1 and Fig. 2a, movie S6), S2 cells undergoing MUGs showed a 3:1 bias (n = 8 cells) in segregating single chromatids containing template DNA strands in an asymmetric vs. apparently symmetric fashion (Fig. 1d, e, Movies S4–5 and Table 1).
Discussion
Here, we have established the experimental conditions to generate MUGs in Drosophila S2 cells in culture (Fig. 3). MUGs represent an established model system that helped elucidating a number of important processes in mitosis, such as kinetochore-microtubule interactions, SAC satisfaction and the role of chromosomes/kinetochores in spindle assembly (Wise and Brinkley 1997, O'Connell et al. 2008, O'Connell et al. 2009, Johnson and Wise 2010). The ability of Drosophila S2 cells to preserve condensed DNA morphology during MUGs allowed the unequivocal tracking of single chromatids (Fig. 1b–e, Movies S2–5) containing template DNA strands, which is an advantage relative to mammalian systems where DNA condensation during MUGs is lost.
Model of MUGs upon Dup depletion in Drosophila S2 cells. Model illustrates possible biased/asymmetric segregation of chromosomes in Dup-depleted cells in comparison to control. a In control cells chromatids equally segregate towards the mitotic poles. b Since Dup-depleted S2 cells only contain template DNA this system/strategy might be useful to investigate biased/asymmetric segregation of DNA strands
Although S2 cells are thought to divide in a symmetrical fashion, there are inherent asymmetries that are normally neglected. This is the case for the mother and daughter centrosomes, which will be inherited by the two daughter cells. The template and replicated DNA strands are also inherently asymmetric, in the sense that one is older than the other, but whether they segregate asymmetrically in this system remains unknown. Moreover, even if they do segregate asymmetrically, there is no obvious reasoning for such behaviour aside from the potential conservation of mechanisms that maybe present in the stem cells from which S2 cells derived. Importantly, it is possible to induce polarized division in S2 cells in culture, for example through the ectopic expression of aPKC or Pins to cell–cell contact sites (Johnston et al. 2009). The powerful and flexible experimental tools together with the simple cytology of S2 cells makes this system potentially suited for the dissection of the molecular mechanism behind asymmetric DNA strand segregation, namely through the combination of high-resolution live cell imaging with loss-of-function studies.
Although beyond the scope of this work, we did notice that those S2 cell MUGs that were able to exit mitosis showed a 3:1 bias in asymmetric vs. apparently symmetric segregation of single chromatids containing template DNA strands. This was surprising in light of previous studies in CHO cells where it was shown that during MUGs small centromere-kinetochore fragments segregate evenly to the daughter cells without showing any strong bias or asymmetry (Johnson and Wise 2010). However, previous studies did detect two populations of CHO cells undergoing MUGs where some showed apparently equal segregation and others where segregation was uneven, depending on whether they had near “diploid” kinetochore numbers (Brinkley et al. 1988). In these cases, the reason for discrepancy might lie in the detection method of small kinetochore fragments, which in S2 cell MUGs does not represent a problem since entire chromatids can be tracked. Are these results in S2 cell MUGs relevant to the “immortal strand” hypothesis? Maybe not, and our results should be taken with caution given that just a small population of cells was analysed in the present study. Moreover, the observed bias might be due to many other mechanisms unrelated to template strand bias, such as asymmetry in the initial distribution of chromatids towards one of the two centrosomes or unequal microtubule nucleation capacity of centrosomes, which would bias the capture of a single chromatid by a particular spindle pole. Nevertheless, we do not think this to be the case as during MUGs initial kinetochore-microtubule attachments are unstable and therefore cannot account for the segregation bias observed several hours after alternating chromatid excursions to both spindle poles. This is further supported by the observation that those S2 cells undergoing MUGs that showed a very early bias of chromatids relative to one of the spindle poles immediately upon nuclear envelope breakdown (NEB) did not reveal any obvious asymmetry in their spatial distribution relative to both poles prior to NEB (Table 1). These situations are particularly relevant because they further provide an opportunity to dissect and visualize how the initial interactions between kinetochores from sister chromatids containing template DNA strands and microtubules from the mitotic apparatus are established. Finally, our results do show that there is no absolute bias/asymmetry in the segregation of all template DNA strands and, in the best case scenario, there might only be a segregation bias of template DNA strands from some, but not all, chromosomes. It will be interesting in the future to reproduce these experiments upon induction of polarization and selective labelling of mother vs. daughter centrosomes in S2 cells using photo-conversion of centriolar proteins (Wang et al. 2009, Januschke et al. 2011).
One important aspect that deserves further consideration in systems undergoing MUGs is that kinetochores are not paired and therefore the entire tension/attachment status of chromosomes is likely to be very different from normal cells, which may influence the segregation pattern of chromatids containing template DNA strands. Accordingly, we observed that the attachments between single chromatids and spindle microtubules in Dup-depleted S2 cells are unstable, with chromatids often switching orientation between the two poles. This highly dynamic kinetochore-microtubule interactions and unstable attachments likely result from the lack of tension in the absence of sister chromatid cohesion and is probably the result of Aurora B-mediated corrections of improper kinetochore-microtubule attachments (e.g. merotelic or monotelic) (Oliveira et al. 2010). Indeed, the majority of Dup-depleted chromatids are scattered around the mitotic spindle and only some are able to align to the spindle equator apparently through the establishment of merotelic attachments, similar to what has been reported in human cells undergoing MUGs (O'Connell et al. 2008). In these cases, segregation bias was shown to depend on the number of microtubules associated between pole and the merotelic kinetochore, favouring segregation towards the pole with the higher number of attached microtubules (Cimini et al. 2003, 2004).
Interestingly, all recorded S2 cells undergoing MUGs exited mitosis but took about three to four times longer than control S2 cells (Table 1). This contrasts with results in Drosophila embryos mutant for Dup, which arrested in mitosis as a result of activated SAC, with stabilized mitotic cyclins and Bub1 kinase present on kinetochores (Garner et al. 2001). On the other hand, mammalian cells undergoing MUGs were able to satisfy the SAC and exited from mitosis (O'Connell et al. 2008), although BubR1 levels on kinetochores were still high. These differences between systems undergoing MUGs indicate that the detailed mechanism of SAC satisfaction/mitotic exit remains to be elucidated and likely involves structural modifications within the kinetochore itself in addition to centromere stretching (Maresca and Salmon 2009, Uchida et al. 2009).
Our results in S2 cells in culture mirror previous experiments with Drosophila embryos mutant for Dup (Whittaker et al. 2000, Parry et al. 2003). This provides an important advantage over in vitro-limited HU/caffeine induced MUGs in mammalian cells in culture to investigate biased/asymmetric DNA strand segregation in vivo using the powerful genetic tools of Drosophila, including the analysis of hypomorphic mutations, in vivo RNAi and clonal cell analysis in specific tissues.
Abbreviations
- MUGs:
-
Mitosis with unreplicated genomes
- Dup:
-
Double parked
- HU:
-
Hydroxyurea
- SAC:
-
Spindle assembly checkpoint
- NEB:
-
Nuclear envelope breakdown
References
Armakolas A, Klar AJ (2006) Cell type regulates selective segregation of mouse chromosome 7 DNA strands in mitosis. Science 311:1146–1149
Armakolas A, Koutsilieris M, Klar AJ (2010) Discovery of the mitotic selective chromatid segregation phenomenon and its implications for vertebrate development. Curr Opin Cell Biol 22:81–87
Brinkley BR, Zinkowski RP, Mollon WL, Davis FM, Pisegna MA, Pershouse M, Rao PN (1988) Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature 336:251–254
Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255:197–200
Charville GW, Rando TA (2011) Stem cell ageing and non-random chromosome segregation. Philos Trans R Soc B Biol Sci 366:85–93
Cimini D, Cameron LA, Salmon ED (2004) Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr Biol 14:2149–2155
Cimini D, Moree B, Canman JC, Salmon ED (2003) Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci 116:4213–4225
Conboy MJ, Karasov AO, Rando TA (2007) High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol 5:e102
Escobar M, Nicolas P, Sangar F, Laurent-Chabalier S, Clair P, Joubert D, Jay P, Legraverend C (2011) Intestinal epithelial stem cells do not protect their genome by asymmetric chromosome segregation. Nat Commun 2:258
Garner M, van Kreeveld S, Su TT (2001) mei-41 and bub1 block mitosis at two distinct steps in response to incomplete DNA replication in Drosophila embryos. Curr Biol: CB 11:1595–1599
Januschke J, Llamazares S, Reina J, Gonzalez C (2011) Drosophila neuroblasts retain the daughter centrosome. Nat Commun 2:243
Johnson MK, Wise DA (2010) Distribution of kinetochore fragments during mitosis with unreplicated genomes. Cytoskeleton (Hoboken) 67:172–177
Johnston CA, Hirono K, Prehoda KE, Doe CQ (2009) Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell 138:1150–1163
King JM, Nicklas RB (2000) Tension on chromosomes increases the number of kinetochore microtubules but only within limits. J Cell Sci 113(Pt 21):3815–3823
Lark KG, Consigli RA, Minocha HC (1966) Segregation of sister chromatids in mammalian cells. Science 154:1202–1205
Lew DJ, Burke DJ, Dutta A (2008) The immortal strand hypothesis: how could it work? Cell 133:21–23
Maiato H, Lince-Faria M (2010) The perpetual movements of anaphase. Cell Mol Life Sci: CMLS 67:2251–2269
Maiato H, Rieder CL, Khodjakov A (2004) Kinetochore-driven formation of kinetochore fibers contributes to spindle assembly during animal mitosis. J Cell Biol 167:831–840
Maiato H, Sunkel CE, Earnshaw WC (2003) Dissecting mitosis by RNAi in Drosophila tissue culture cells. Biol Proced Online 5:153–161
Maresca TJ, Salmon ED (2009) Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol 184:373–381
Moutinho-Pereira S, Matos I, Maiato H (2010) Drosophila S2 cells as a model system to investigate mitotic spindle dynamics, architecture, and function. Methods Cell Biol 97:243–257
O'Connell CB, Loncarek J, Hergert P, Kourtidis A, Conklin DS, Khodjakov A (2008) The spindle assembly checkpoint is satisfied in the absence of interkinetochore tension during mitosis with unreplicated genomes. J Cell Biol 183:29–36
O'Connell CB, Loncarek J, Kalab P, Khodjakov A (2009) Relative contributions of chromatin and kinetochores to mitotic spindle assembly. J Cell Biol 187:43–51
Oliveira RA, Hamilton RS, Pauli A, Davis I, Nasmyth K (2010) Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat Cell Biol 12:185–192
Parry DH, Hickson GR, O'Farrell PH (2003) Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr Biol: CB 13:647–653
Pinsky BA, Biggins S (2005) The spindle checkpoint: tension versus attachment. Trends Cell Biol 15:486–493
Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S (2012) A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148:112–125
Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H (2011) Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 30:1104–1109
Tajbakhsh S (2008) Stem cell identity and template DNA strand segregation. Curr Opin Cell Biol 20:716–722
Tajbakhsh S, Gonzalez C (2009) Biased segregation of DNA and centrosomes: moving together or drifting apart? Nat Rev Mol Cell Biol 10:804–810
Uchida KS, Takagaki K, Kumada K, Hirayama Y, Noda T, Hirota T (2009) Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol 184:383–390
Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461:947–955
Whittaker AJ, Royzman I, Orr-Weaver TL (2000) Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev 14:1765–1776
Wise DA, Brinkley BR (1997) Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell Motil Cytoskeleton 36:291–302
Acknowledgments
We would like to thank António Pereira for insightful discussions on chromosome segregation in MUGs. We also acknowledge the GABBA PhD Program for support to D.D and D.P. Work in the laboratory of H.M. is funded by grants PTDC/SAU-GMG/099704/2008 and PTDC/SAU-ONC/112917/2009 from FCT (COMPETE-FEDER), the Human Frontier Science Program and the 7th framework program grant PRECISE from the European Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yves Barral.
Danica Drpic and Marin Barisic contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary movies S1–S5
Live cell imaging of control (movie S1) and Dup-depleted Drosophila S2 cells (movies S2–S5) stably expressing GFP-H2B (showed in green) and mCherry-α-tubulin (showed in red). Scale bar 5 μm. Time is in h:min. (MOV 396 kb)
Supplementary movies S6–S8
Live cell imaging of control (movie S6) and Dup-depleted Drosophila S2 cells (movies S7 and S8) stably expressing GFP-α-tubulin (showed in red) and CID-mCherry (showed in green). Scale bar, 5 μm. Time is in h:min. (MOV 636 kb)
Rights and permissions
About this article
Cite this article
Drpic, D., Barisic, M., Pinheiro, D. et al. Selective tracking of template DNA strands after induction of mitosis with unreplicated genomes (MUGs) in Drosophila S2 cells. Chromosome Res 21, 329–337 (2013). https://doi.org/10.1007/s10577-013-9354-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-013-9354-z