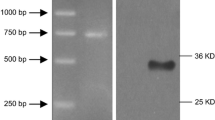
Abstract
Bone marrow stromal cells (BMSCs) are attractive cellular sources for cell therapy of many diseases, specifically neurodegenerative ones. The potential capability of BMSCs could be further augmented by enhancing their neuroprotective property, differentiation potential, and survival rate subsequent to transplantation. Therefore, a concurrent upregulation of neurotrophin-3 (NT-3) and its high affinity receptor, tyrosin kinase C (TrkC), was utilized in our study. BMSCs were cotransfected with pDsRed1-N1-NT-3 and pCMX-TrkC plasmids before induction of neural differentiation. pEGFP-N1-transfected BMSCs were also employed as a control. Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was employed for gene expression analysis. Cell viability was evaluated by MTT assay, while apoptosis rate was assessed by flow cytometry after PI and Annexin V staining. NT-3 and TrkC mRNA levels were greatly elevated following cotransfection of cells with pDsRed1-N1-NT-3 and pCMX-TrkC vectors. The expression of neural markers (i.e., NFM, and NeuroD1) was augmented in cotransfected BMSCs, compared to the control ones, after neural induction. At each time point, the viability and apoptosis rates of the cells over-expressing NT-3 and TrkC showed increased and reduced patterns, respectively. Our data demonstrated that NT-3/TrkC-co-transfected BMSCs, compared to those of intact cells, could be more beneficial graft candidates for the upcoming treatment strategies of neurogenic disorders due to their increased viability and expression of neural markers. This may be due to their increased level of neural differentiation potential and/or their enhanced rate of survival and/or their useful capacity to secrete NT-3.





Similar content being viewed by others
References
Blesch A (2006) Neurotrophic factors in neurodegeneration. Brain Pathol 16:295–303
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–347
Chen Q et al (2005) Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res 80:611–619
Cheng L, Saphieha P, Kittlerova P, Hauswirth WW, Di Polo AT (2002) TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci 22:3977–3986
Davies AM, Minichiello L, Klein R (1995) Developmental changes in NT-3 signalling via TrkA and TrkB in embryonic neurons. EMBO J 14:4482–4489
Edalat H, Hajebrahimi Z, Movahedin M, Tavallaei M, Amiri S, Mowla SJ (2011) p75NTR suppression in rat bone marrow stromal stem cells significantly reduced their rate of apoptosis during neural differentiation. Neurosci Lett 498:15–19
Edalat H, Hajebrahimi Z, Pirhajati V, Movahedin M, Tavallaei M, Soroush MR, Mowla SJ (2013) Transplanting p75-suppressed bone marrow stromal cells promotes functional behavior in a rat model of spinal cord injury. Iran Biomed J 17:140–145
Farhadi HF, Mowla SJ, Petrecca K, Morris SJ, Seidah NG, Murphy RA (2000) Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J Neurosci 20:4059–4068
Gao Z et al (2009) Neurodl is essential for the survival and maturation of adult-born neurons. Nat Neurosci 12:1090–1092
Hajebrahimi Z, Mowla SJ, Movahedin M, Tavallaei M (2008) Gene expression alterations of neurotrophins, their receptors and prohormone convertases in a rat model of spinal cord contusion. Neurosci Lett 441:261–266
Hantzopoulos PA, Suri C, Glass DJ, Goldfarb MP, Yancopoulos GD (1994) The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron 13:187–201
Hokari M, Kuroda S, Shichinohe H, Yano S, Hida K, Iwasaki Y (2008) Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J Neurosci Res 86:1024–1035
Ip NY et al (1993) Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron 10:137–149
Jafarnejad SM, Mowla SJ, Matin MM (2008) Knocking-down the expression of nucleostemin significantly decreases rate of proliferation of rat bone marrow stromal stem cells in an apparently p53-independent manner. Cell Prolif 41:28–35
Verge VM, Gratto KA, Karchewski LA, Richardson PM (1996) Neurotrophins and nerve injury in the adult. Philos Trans R Soc Lond B 351:423–430
Yaghoobi MM, Mowla SJ (2006) Differential gene expression pattern of neurotrophins and their receptors during neuronal differentiation of rat bone marrow stromal cells. Neurosci Lett 397:149–154
Yaghoobi MM, Mowla SJ, Tiraihi T (2005) Nucleostemin, a coordinator of self-renewal, is expressed in rat marrow stromal cells and turns off after induction of neural differentiation. Neurosci Lett 390:81–86
Zhang W, Zeng YS, Zhang XB, Wang JM, Zhang W, Chen SJ (2006) Combination of adenoviral vector-mediated neurotrophin-3 gene transfer and retinoic acid promotes adult bone marrow cells to differentiate into neuronal phenotypes. Neurosci Lett 408:98–103
Zhang YQ, Zeng X, He LM, Ding Y, Li Y, Zeng YS (2010) NT-3 gene modified Schwann cells promote TrkC gene modified mesenchymal stem cells to differentiate into neuron-like cells in vitro. Anat Sci Int 85:61–67
Acknowledgements
We are grateful to Dr. Phil Barker for kindly providing us the plasmids. This research was supported by a grant from Basij Elmi Organization (Grant No. 3657).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
We certify that all applicable institutional regulations concerning the ethical use of animals were followed during the course of this research.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Edalat, H., Hajebrahimi, Z., Pirhajati, V. et al. Exogenous Expression of Nt-3 and TrkC Genes in Bone Marrow Stromal Cells Elevated the Survival Rate of the Cells in the Course of Neural Differentiation. Cell Mol Neurobiol 37, 1187–1194 (2017). https://doi.org/10.1007/s10571-016-0448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0448-y