Abstract
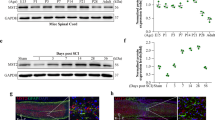
Reactive astrogliosis and microgliosis after spinal cord injury (SCI) contribute to glial scar formation that impedes axonal regeneration. The mechanisms underlying reactive astrocyte and microglia proliferation upon injury remain partially understood. Peroxiredoxin 1 (PRDX1) is an antioxidant participating in cell proliferation, differentiation, and apoptosis. However, PRDX1 functions in SCI-induced astrocyte and microglia proliferation are unknown. In this study, we established an acute spinal cord contusion injury model in adult rats to investigate the potential role of PRDX1 during the pathological process of SCI. We found the palpable expression increase of PRDX1 after SCI by western blot and immunohistochemistry staining. Double immunofluorescence staining showed that PRDX1 expression mainly increased in astrocytes and microglia. In addition, PRDX1/proliferating cell nuclear antigen (PCNA) colocalized in astrocytes and microglia. Furthermore, PCNA expression also elevated after SCI, as well as was positively correlated with PRDX1 expression. In vitro, PRDX1 expression in primary rat spinal cord astrocytes and microglia changed in a concentration- and time-dependent manner according to LPS treatment. In addition, PRDX1 knockdown in astrocytes and microglia resulted in the decrease of PCNA expression after LPS stimulation, showing that PRDX1 promoted astrocyte and microglia proliferation after inflammation. Our results suggested that PRDX1 might play a crucial role in astrocyte and microglia proliferation after SCI.







Similar content being viewed by others
References
Bao Y, Qin L, Kim E, Bhosle S, Guo H, Febbraio M, Haskew-Layton RE, Ratan R, Cho S (2012) CD36 is involved in astrocyte activation and astroglial scar formation. J Cereb Blood Flow Metab 32(8):1567–1577
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Becher B, Prat A, Antel JP (2000) Brain-immune connection: immuno-regulatory properties of CNS-resident cells. Glia 29(4):293–304
Castriotta RJ, Murthy JN (2009) Hypoventilation after spinal cord injury. Semin Respir Crit Care Med 30(3):330–338
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209(2):294–301
Gruner JA (1992) A monitored contusion model of spinal cord injury in the rat. J Neurotrauma 9(2):123–126; discussion 126–8
Hayakawa K, Okazaki R, Morioka K, Nakamura K, Tanaka S, Ogata T (2014) Lipopolysaccharide preconditioning facilitates M2 activation of resident microglia after spinal cord injury. J Neurosci Res 92(12):1647–1658
Ishii H, Tanabe S, Ueno M, Kubo T, Kayama H, Serada S, Fujimoto M, Takeda K, Naka T, Yamashita T (2013) ifn-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell Death Dis 4:e710
Jin MH, Lee YH, Kim JM, Sun HN, Moon EY, Shong MH, Kim SU, Lee SH, Lee TH, Yu DY, Lee DS (2005) Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci Lett 381(3):252–257
Kang SW, Baines IC, Rhee SG (1998) Characterization of a mammalian peroxiredoxin that contains one conserved cysteine. J Biol Chem 273(11):6303–6311
Khalatbary AR, Zarrinjoei GR (2012) Anti-inflammatory effect of oleuropein in experimental rat spinal cord trauma. Iran Red Crescent Med J 14(4):229–234
Kim SU, Park YH, Min JS, Sun HN, Han YH, Hua JM, Lee TH, Lee SR, Chang KT, Kang SW, Kim JM, Yu DY, Lee SH, Lee DS (2013) Peroxiredoxin I is a ROS/p38 MAPK-dependent inducible antioxidant that regulates NF-kappaB-mediated iNOS induction and microglial activation. J Neuroimmunol 259(1–2):26–36
Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA (2003) Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424(6948):561–565
Reis e Sousa C (2004) Toll-like receptors and dendritic cells: for whom the bug tolls. Semin Immunol 16(1):27–34
Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER (1999) A family of novel peroxidases, peroxiredoxins. Biofactors 10(2–3):207–209
Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol 17(2):183–189
Riddell JR, Bshara W, Moser MT, Spernyak JA, Foster BA, Gollnick SO (2011) Peroxiredoxin 1 controls prostate cancer growth through toll-like receptor 4-dependent regulation of tumor vasculature. Cancer Res 71(5):1637–1646
Rolls A, Shechter R, Schwartz M (2009) The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10(3):235–241
Saadoun S, Bell BA, Verkman AS, Papadopoulos MC (2008) Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain 131(Pt 4):1087–1098
Seo MS, Kang SW, Kim K, Baines IC, Lee TH, Rhee SG (2000) Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem 275(27):20346–20354
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647
Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P (2014) Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain 137(Pt 3):707–723
Wu J, Pajoohesh-Ganji A, Stoica BA, Dinizo M, Guanciale K, Faden AI (2012) Delayed expression of cell cycle proteins contributes to astroglial scar formation and chronic inflammation after rat spinal cord contusion. J Neuroinflammation 9:169
Yaguchi M, Ohta S, Toyama Y, Kawakami Y, Toda M (2008) Functional recovery after spinal cord injury in mice through activation of microglia and dendritic cells after IL-12 administration. J Neurosci Res 86(9):1972–1980
Yan Y, Sabharwal P, Rao M, Sockanathan S (2009) The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2. Cell 138(6):1209–1221
Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, Sun SK, Luo ZJ (2012) Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One 7(6):e38381
Zhang J, Cui Z, Feng G, Bao G, Xu G, Sun Y, Wang L, Chen J, Jin H, Liu J, Yang L, Li W (2015) RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. Int J Biochem Cell Biol 60:43–52
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16(2):109–110
Conflict of interests
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shen Huang, Xiaojuan Liu, and Jinlong Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, S., Liu, X., Zhang, J. et al. Expression of Peroxiredoxin 1 After Traumatic Spinal Cord Injury in Rats. Cell Mol Neurobiol 35, 1217–1226 (2015). https://doi.org/10.1007/s10571-015-0214-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-015-0214-6