Abstract
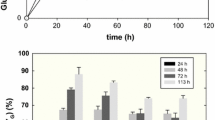
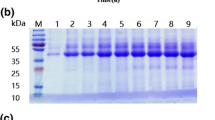
The carbohydrate-binding modules (CBMs) have emerged as an interesting alternative to enzymes for fibers modification, e.g. of pulp and paper. Glycosylation in CBMs is thought to have a key role in the improvement of cellulose fibers. Thus, in this work the non-glycosylated (CBM3mt) and glycosylated (CBM3wt) recombinant versions of CBM3 from Clostridium thermocellum CipA—both produced in Pichia pastoris—were studied. Binding assays showed that CBM3mt had a higher affinity for microcrystalline cellulose (Avicel) than CBM3wt. In addition, CBM3mt produced a much higher hydrophobization of Whatman paper than CBM3wt. However, the effects of the two CBM3s on pulp and paper were identical. The CBM3s did not affect the drainability of Eucalyptus globulus or a mixture of E. globulus and Pinus sylvestris pulps. On the other hand, both improved significantly strength-related properties of E. globulus papersheets, namely burst (up to 12 %) and tensile strength (up to 10 %) indexes. This is the first report showing the capacity of CBM3 from C. termocellum CipA to modify paper properties. The results showed that glycosylation did not influence the drainage of CBM3-treated pulps nor the properties of the produced papers. Thus, glycans in glycosylated CBM3 may not be related with fiber improvement, namely superior pulp drainage.






Similar content being viewed by others
References
Bager R, Johansen JS, Jensen JK, Stensballe A, Jendroszek A, Buxbom L et al (2013) Protein conformational change delayed by steric hindrance from an N-linked glycan. J Mol Biol 425:2867–2877
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Blanchard V, Gadkari RA, Gerwig GJ, Leeflang BR, Dighe RR, Kamerling JP (2007) Characterization of the N-linked oligosaccharides from human chorionic gonadotropin expressed in the methylotrophic yeast Pichia pastoris. Glycoconj J 24:33–47
Bohne-Lang A, von der Lieth CW (2005) GlyProt: in silico glycosylation of proteins. Nucleic Acids Res 33:W214–W219
Boraston AB, McLean BW, Guarna MM, Amandaron-Akow E, Kilburn DG (2001a) A family 2a carbohydrate-binding module suitable as an affinity tag for proteins produced in Pichia pastoris. Protein Expr Purif 21:417–423
Boraston AB, Warren RAJ, Kilburn DG (2001b) Glycosylation by Pichia pastoris decreases the affinity of a family 2a carbohydrate-binding module from Cellulomonas fimi: a functional and mutational analysis. Biochem J 358:423–430
Boraston AB, Sandercock L, Warren RA, Kilburn DG (2003) O-glycosylation of a recombinant carbohydrate-binding module mutant secreted by Pichia pastoris. J Mol Microbiol Biotechnol 5(1):29–36
Cadena EM, Chriac AI, Pastor FIJ, Diaz P, Vidal T, Torres AL (2010) Use of cellulases and recombinant cellulose binding domains for refining TCF kraft pulp. Biotechnol Prog 26:960–967
Chen LQ, Drake MR, Resch MG, Greene ER, Himmel ME, Chaffey PK et al (2014) Specificity of O-glycosylation in enhancing the stability and cellulose binding affinity of Family 1 carbohydrate-binding modules. Proc Natl Acad Sci USA 111:7612–7617
Gao SH, You C, Renneckar S, Bao J, Zhang YHP (2014) New insights into enzymatic hydrolysis of heterogeneous cellulose by using carbohydrate-binding module 3 containing GFP and carbohydrate- binding module 17 containing CFP. Biotechnol Biofuels 7:24
Gomes D, Rodrigues AC, Domingues L, Gama FM (2015) Cellulase recycling in biorefineries: is it possible? Appl Microbiol Biotechnol 99:4131–4143
Hong J, Yea X, Wang Y, Zhang YH (2008) Bioseparation of recombinant cellulose-binding module-proteins by affinity adsorption on an ultra-high-capacity cellulosic adsorbent. Anal Chim Acta 621:193–199
Humphrey W, Dalke A, Schulten K (1996) VMD—visual molecular dynamics. J Molec Graphics 14:33–38
Kim DW, Jang YH, Kim CS, Lee NS (2001) Effect of metal ions on the degradation and adsorption of two cellobiohydrolases on microcrystalline cellulose. Bull Korean Chem Soc 22:716–720
Kitaoka T, Tanaka H (2001) Novel paper strength additive containing cellulose-binding domain of cellulase. J Wood Sci 47:322–324
Lemos MA, Teixeira JA, Mota M, Gama FM (2000) A simple method to separate cellulose-binding domains of fungal cellulases after digestion by a protease. Biotechnol Lett 22:703–707
Levy I, Nussinovitch A, Shpigel E, Shoseyov O (2002) Recombinant cellulose crosslinking protein: a novel paper-modification biomaterial. Cellulose 9:91–98
Levy I, Paldi T, Shoseyov O (2004) Engineering a bifunctional starch-cellulose cross-bridge protein. Biomaterials 25:1841–1849
Lombard V, Ramulu HG, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495
Machado J, Araujo A, Pinto R, Gama FM (2009) Studies on the interaction of the carbohydrate binding module 3 from the Clostridium thermocellum CipA scaffolding protein with cellulose and paper fibres. Cellulose 16:817–824
Montesino R, Nimtz M, Quintero O, Garcia R, Falcón V, Cremata JA (1999) Characterization of the oligosaccharides assembled on the Pichia pastoris–expressed recombinant aspartic protease. Glycobiology 9:1037–1043
Oliveira C, Felix W, Moreira RA, Teixeira JA, Domingues L (2008) Expression of frutalin, an alpha-D-galactose-binding jacalin-related lectin, in the yeast Pichia pastoris. Protein Expr Purif 60:188–193
Oliveira C, Carvalho V, Domingues L, Gama FM (2015) Recombinant CBM-fusion technology: applications overview. Biotechnol Adv 33:358–369
Pala H, Lemos MA, Mota M, Gama FM (2001) Enzymatic upgrade of old paperboard containers. Enzyme Microb Tech 29:274–279
Payne CM, Resch MG, Chen L, Crowley MF, Himmel ME, Taylor LE 2nd et al (2013) Glycosylated linkers in multimodular lignocellulose-degrading enzymes dynamically bind to cellulose. Proc Natl Acad Sci USA 110:14646–14651
Pinto R (2006) Production of cellulose-binding domains by proteolysis; studies on the adsorption and modification of cellulose fibres. Ph.D. thesis, University of Minho, Braga
Pinto R, Moreira S, Mota M, Gama M (2004a) Studies on the cellulose-binding domains adsorption to cellulose. Langmuir 20:1409–1413
Pinto R, Amaral E, Costa AP, Gama FM, Duarte AP (2004b) Improving papermaking with cellulose-binding domains. CIADICYP 2004: congresso Iberoamericano de Investigacion en Cellulose y Papel, vol. 2004. Córdoba, Spain, pp 303–305. ISBN 84-7498-504-8
Pinto R, Carvalho J, Mota M, Gama M (2006) Large-scale production of cellulose-binding domains. Adsorption studies using CBD-FITC conjugates. Cellulose 13:557–569
Shi XR, Zheng F, Pan RH, Wang J, Ding SJ (2014) Engineering and comparative characteristics of double carbohydrate binding modules as a strength additive for papermaking applications. Bioresources 9:3117–3131
Taylor CB, Talib MF, McCabe C, Bu L, Adney WS, Himmel ME et al (2012) Computational investigation of glycosylation effects on a family 1 carbohydrate-binding module. J Biol Chem 287:3147–3155
Tormo J, Lamed R, Chirino AJ, Morag E, Bayer EA, Shoham Y et al (1996) Crystal structure of a bacterial family-III cellulose-binding domain: a general mechanism for attachment to cellulose. EMBO J 15:5739–5751
Wan W, Wang DM, Gao XL, Hong J (2011) Expression of family 3 cellulose-binding module (CBM3) as an affinity tag for recombinant proteins in yeast. Appl Microbiol Biotechnol 91:789–798
Yokota S, Matsuo K, Kitaoka T, Wariishi H (2009) Retention and paper-strength characteristics of anionic polyacrylamides conjugated with carbohydrate-binding modules. Bioresources 4:234–244
Acknowledgments
C. Oliveira acknowledges support from Fundação para a Ciência e a Tecnologia (FCT), Portugal (Grant SFRH/BDP/63831/2009). The authors thank the FCT GlycoCBMs Project REF. PTDC/AGR-FOR/3090/2012—FCOMP-01-0124-FEDER-027948, the FCT Strategic Project PEst-OE/EQB/LA0023/2013, and the Project “BioInd—Biotechnology and Bioengineering for improved Industrial and Agro-Food processes”, REF. NORTE-07-0124-FEDER-000028 Co-funded by the Programa Operacional Regional do Norte (ON.2—O Novo Norte), QREN, FEDER.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira, C., Sepúlveda, G., Aguiar, T.Q. et al. Modification of paper properties using carbohydrate-binding module 3 from the Clostridium thermocellum CipA scaffolding protein produced in Pichia pastoris: elucidation of the glycosylation effect. Cellulose 22, 2755–2765 (2015). https://doi.org/10.1007/s10570-015-0655-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0655-6