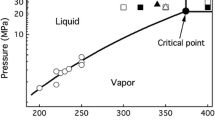
Abstract
Subcritical and supercritical water are known to dissolve crystalline cellulose, offering a simple way to produce low molar mass cellulose which precipitates at ambient temperatures. However, the yield of precipitate is limited by concomitant degradation reactions. In this study, the formation of cellulose precipitate from six different microcrystalline celluloses (MCC) was investigated in 0.2 s treatments at 250–380 °C. The results were elucidated with a simple two-step kinetic model. The maximum yield of cellulose precipitate depended on the choice of starting material, which had a more important role than the optimized treatment time or temperature. Wood-derived MCCs dissolved faster and resulted in a higher yield of precipitate than those prepared from cotton linter. The highest yield of precipitate, 68 %, was recovered from a MCC prepared from mercerized prehydrolysis hardwood Kraft pulp. The high yield of precipitate could not be attributed to any single factor but in general the wood-derived MCCs had a smaller particle size, smaller crystallite dimensions, and lower molar mass than their cotton-derived equivalents. The analysis of molar mass distributions indicated heterogeneous dissolution mechanism at 250 and 320 °C whereas at 380 °C the cellulose crystallites were subjected to random chain cleavage.










Similar content being viewed by others
References
Abdullah R, Ueda K, Saka S (2014) Hydrothermal decomposition of various crystalline celluloses as treated by semi-flow hot-compressed water. J Wood Sci 60:278–286. doi:10.1007/s10086-014-1401-7
Adschiri T, Hirose S, Malaluan R, Arai K (1993) Noncatalytic conversion of cellulose in supercritical and subcritical water. J Chem Eng Jpn 26:676–680. doi:10.1252/jcej.26.676
Ballauff M, Wolf BA (1981) Degradation of chain molecules. 1. Exact solution of the kinetic equations. Macromolecules 14:654–658. doi:10.1021/ma50004a039
Bergenstråhle M, Wohlert J, Himmel ME, Brady JW (2010) Simulation studies of the insolubility of cellulose. Carbohydr Res 345:2060–2066. doi:10.1016/j.carres.2010.06.017
Blumberg RL, Stanley HE, Geiger A, Mausbach P (1984) Connectivity of hydrogen bonds in liquid water. J Chem Phys 80:5230–5241. doi:10.1063/1.446593
Borrega M, Tolonen LK, Bardot F, Testova L, Sixta H (2012) Potential of hot water extraction of birch wood to produce high-purity dissolving pulp after alkaline pulping. Bioresour Technol 135:665–671. doi:10.1016/j.biortech.2012.11.107
Cantero DA, Bermejo MD, Cocero MJ (2012a) High glucose selectivity in pressurized water hydrolysis of cellulose using ultra-fast reactors. Bioresour Technol 135:697–703. doi: 10.1016/j.biortech.2012.09.035
Cantero DA, Bermejo MD, Cocero MJ (2012b) Kinetic analysis of cellulose depolymerization reactions in near critical water. J Supercrit Fluids 75:48–57. doi:10.1016/j.supflu.2012.12.013
Chandler D (2005) Interfaces and the driving force of hydrophobic assembly. Nature 437:640–647. doi:10.1038/nature04162
Deguchi S, Tsujii K, Horikoshi K (2008) Crystalline-to-amorphous transformation of cellulose in hot and compressed water and its implications for hydrothermal conversion. Green Chem 10:191–196. doi:10.1039/B713655B
Ehara K, Saka S (2002) A comparative study on chemical conversion of cellulose between the batch-type and flow-type systems in supercritical water. Cellulose 9:301–311. doi:10.1023/A:1021192711007
Emsley AM, Heywood RJ (1995) Computer modelling of the degradation of linear polymers. Polym Degrad Stab 49:145–149. doi:10.1016/0141-3910(95)00067-V
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca, New York
Gorbaty YE, Kalinichev A (1995) Hydrogen bonding in supercritical water. 1. Experimental results. J Phys Chem 99:5336–5340. doi:10.1021/j100015a016
Gross AS, Chu J (2010) On the molecular origins of biomass recalcitrance: the interaction network and solvation structures of cellulose microfibrils. J Phys Chem B 114:13333–13341. doi:10.1021/jp106452m
Gross AS, Bell AT, Chu J (2012) Entropy of cellulose dissolution in water and in the ionic liquid 1-butyl-3-methylimidazolim chloride. Phys Chem Chem Phys 14:8425–8430. doi:10.1039/C2CP40417F
Himmel ME, Ding S, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315:804–807. doi:10.1126/science.1137016
Hodes M, Marrone PA, Hong GT, Smith KA, Tester JW (2004) Salt precipitation and scale control in supercritical water oxidation—Part A: fundamentals and research. J Supercrit Fluids 29:265–288. doi:10.1016/S0896-8446(03)00093-7
Jollet V, Chambon F, Rataboul F, Cabiac A, Pinel C, Guillon E, Essayem N (2009) Non-catalyzed and Pt/γ-Al2O3 -catalyzed hydrothermal cellulose dissolution-conversion: influence of the reaction parameters and analysis of the unreacted cellulose. Green Chem 11:2052–2060. doi:10.1039/B915758A
Kabyemela BM, Adschiri T, Malaluan RM, Arai K (1997) Kinetics of glucose epimerization and decomposition in subcritical and supercritical water. Ind Eng Chem Res 36:1552–1558. doi:10.1021/ie960250h
Klemm D, Heublein B, Fink H, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393. doi:10.1002/anie.200460587
Kruse A, Dinjus E (2007) Hot compressed water as reaction medium and reactant. Properties and synthesis reactions. J Supercrit Fluids 39:362–380. doi:10.1016/j.supflu.2006.03.016
Lindman B, Karlström G, Stigsson L (2010) On the mechanism of dissolution of cellulose. J Mol Liq 156:76–81. doi:10.1016/j.molliq.2010.04.016
Marshall WL, Franck EU (1981) Ion product of water substance, 0–1000 & #xB0;C, 1–10,000 bars. New international formulation and its background. J Phys Chem Ref Data 10:295–304. doi:10.1063/1.555643
McKeown JJ, Lyness WI (1960) Evidence of retention of the crystalline-amorphous ratio of cellulose during heterogeneous acid hydrolysis. J Polym Sci 47:9–17. doi:10.1002/pol.1960.1204714902
Millett MA, Moore WE, Saeman JF (1954) Preparation and properties of hydrocelluloses. Ind Eng Chem 46:1493–1497. doi:10.1021/ie50535a051
Miyamoto H, Abdullah R, Tokimura H, Hayakawa D, Ueda K, Saka S (2014) Molecular dynamics simulation of dissociation behavior of various crystalline celluloses treated with hot-compressed water. Cellulose. doi:10.1007/s10570-014-0343-y
Moiseev YV, Khalturinskii NA, Zaikov GE (1976) The mechanism of the acid-catalyzed hydrolysis of glucosides. Carbohydr Res 51:23–37
Möller M, Harnisch F, Schröder U (2013) Hydrothermal liquefaction of cellulose in subcritical water—the role of crystallinity on the cellulose reactivity. Rsc Advances 3:11035–11044. doi:10.1039/C3RA41582A
Mussato SL, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohydr Polym 68:587–597. doi:10.1016/j.carbpol.2006.12.011
Nelson ML (1960) Apparent activation energy of hydrolysis of some cellulosic materials. J Polym Sci. 43:351–371. doi:10.1002/pol.1960.1204314207
Nevell TP, Upton WR (1976) The hydrolysis of cotton cellulose by hydrochloric acid in benzene. Carbohydr Res 49:163–174. doi:10.1016/S0008-6215(00)83134-1
Penttilä PA, Kilpeläinen P, Tolonen L, Suuronen J, Sixta H, Willfoer S, Serimaa R (2013) Effects of pressurized hot water extraction on the nanoscale structure of birch sawdust. Cellulose 20:2335–2347. doi:10.1007/s10570-013-0001-9
Roberfroid MJ (2007) Prebiotics: the concept revisited. J Nutr 137:830S–837S
Roman M, Winter WT (2004) Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 5:1671–1677. doi:10.1021/bm034519+
Saka S, Ueno T (1999) Chemical conversion of various celluloses to glucose and its derivatives in supercritical water. Cellulose 6:177–191. doi:10.1023/A:1009232508644
Sasaki M, Kabyemela B, Malaluan R, Hirose S, Takeda N, Adschiri T, Arai K (1998) Cellulose hydrolysis in subcritical and supercritical water. J Supercrit Fluids 13:261–268. doi:10.1016/S0896-8446(98)00060-6
Sasaki M, Adschiri T, Arai K (2003) Production of cellulose II from native cellulose by near-and supercritical water solubilization. J Agric Food Chem 51:5376–5381. doi:10.1021/jf025989i
Sasaki M, Adschiri T, Arai K (2004) Kinetics of cellulose conversion at 25 MPa in sub- and supercritical water. AIChE J 50:192–202. doi:10.1002/aic.10018
Segal L, Creely J, Martin A, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794. doi:10.1177/004051755902901003
Sharples A (1957) The hydrolysis of cellulose and its relation to structure. Trans Faraday Soc 53:1003–1013. doi:10.1039/TF9575301003
Shen T, Langan P, French AD, Johnson GP, Gnanakaran S (2009) Conformational flexibility of soluble cellulose oligomers: chain length and temperature dependence. J Am Chem Soc 131:14786–14794. doi:10.1021/ja9034158
Southall NT, Dill KA, Haymet A (2002) A view of the hydrophobic effect. J Phys Chem B 106:521–533. doi:10.1021/jp015514e
Taylor J (1957) The water solubilities and heats of solution of short chain cellulosic oligosaccharides. Trans Faraday Soc 53:1198–1203
Testova L, Chong S, Tenkanen M, Sixta H (2011) Autohydrolysis of birch wood. Holzforschung 65:535–542. doi:10.1515/hf.2011.073
Testova L, Borrega M, Tolonen LK, Penttilä PA, Serimaa R, Larsson PT, Sixta H (2014) Dissolving-grade birch pulps produced under various prehydrolysis intensities: quality, structure and applications. Cellulose 21:2007–2021. doi:10.1007/s10570-014-0182-x
Tolonen LK, Zuckerstatter G, Penttilä PA, Milacher W, Habicht W, Serimaa R, Kruse A, Sixta H (2011) Structural changes in microcrystalline cellulose in subcritical water treatment. Biomacromolecules 12:2544–2551. doi:10.1021/bm200351y
Tolonen LK, Penttilä PA, Kruse A, Serimaa R, Sixta H (2013) The swelling and dissolution of cellulose crystallites in subcritical and supercritical water. Cellulose 20:2731–2744. doi:10.1007/s10570-013-0072-7
Tolonen LK, Bergenstråhle-Wohlert M, Sixta H, Wohlert J (2015a) Solubility of cellulose in supercritical water studied by molecular dynamics simulations. J Phys Chem B. doi:10.1021/acs.jpcb.5b01121
Tolonen LK, Juvonen M, Niemelä K, Mikkelson A, Tenkanen M, Sixta H (2015b) Supercritical water treatment for cello-oligosaccharide production from microcrystalline cellulose. Carbohydr Res 401:16–23. doi:10.1016/j.carres.2014.10.012
Uematsu M, Franck EU (1980) Static dielectric constant of water and steam. J Phys Chem Ref Data 9:1291–1306. doi:10.1063/1.555632
Vanhatalo KM, Dahl OP (2014) Effect of mild acid hydrolysis parameters on properties of microcrystalline cellulose. BioResources 9:4729–4740
Weingaertner H, Franck EU (2005) Supercritical water as a solvent. Angew Chem Int Ed 44:2672–2692. doi:10.1002/anie.200462468
Yu Y, Wu H (2009) Characteristics and precipitation of glucose oligomers in the fresh liquid products obtained from the hydrolysis of cellulose in hot-compressed water. Ind Eng Chem Res 48:10682–10690. doi:10.1021/ie900768m
Yu Y, Wu H (2010a) Understanding the primary liquid products of cellulose hydrolysis in hot-compressed water at various reaction temperatures. Energy Fuels 24:1963–1971. doi:10.1021/ef9013746
Yu Y, Wu H (2010b) Evolution of primary liquid products and evidence of in situ structural changes in cellulose with conversion during hydrolysis in hot-compressed water. Ind Eng Chem Res 49:3919–3925
Yu Y, Wu H (2011) Effect of ball milling on the hydrolysis of microcrystalline cellulose in hot-compressed water. AIChE J 57:793–800. doi:10.1002/aic.12288
Acknowledgments
Seppo Jääskeläinen, Terho Konttinen, and the workshop are acknowledged for their work with the reactor system. This work was funded by the Finnish Funding Agency for Technology and Innovations (TEKES), and Finnish Bioeconomy Cluster Ltd. as a part of the Future Biorefinery Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tolonen, L.K., Penttilä, P.A., Serimaa, R. et al. The yield of cellulose precipitate from sub- and supercritical water treatment of various microcrystalline celluloses. Cellulose 22, 1715–1728 (2015). https://doi.org/10.1007/s10570-015-0628-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0628-9