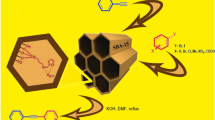
Abstract
The solid supported palladium(0) nanoparticles (NPs) were found as an active heterogeneous catalyst for regioselective hydrosilylation of alkynes with organosilanes in the presence of NaI as additive. Aliphatic as well as aromatic terminal/substituted alkynes with both electron releasing and withdrawing functionalities similarly participated in the hydrosilylation to produce regioselective β-isomers of vinylsilanes under mild reaction conditions. Reducible functional groups such as nitrile, ester, halide, alkene and alkyne were also found to be tolerated under this condition. Furthermore, the triethylsilylstyrene was applied for consecutive iododesilylation followed by Suzuki coupling reaction to produce stilbenes. The air/moisture stable SS–Pd catalyst was recycled for hydrosilylation reaction up to ten runs without significant loss of its catalytic activity.
Graphical Abstract
Solid Supported Palladium(0) Nanoparticles: An Efficient Heterogeneous Catalyst for Regioselective Hydrosilylation of Alkynes and Suzuki Coupling of β-Arylvinyl Iodides






Similar content being viewed by others
References
Ojima I, Li ZY, Zhu JW (1998) In: Rappoport Z, Apeloig Y (eds) Chemistry of organic silicon compounds, Wiley, Chichester, p 1687–1792
Langkopf E, Schinzer D (1995) Chem Rev 95:1375
Hiyama T (1998) In: Diederich F, Stang PJ (eds) Metal-catalyzed cross-coupling reactions, Wiley, Weinheim, p 421
Hiyama T, Shirakawa E (2002) Top Curr Chem 219:61
Hatanaka Y, Hiyama T (1991) Synlett 1991:845
Mowery M, De Shong P (1999) Org Lett 1:2137
Denmark SE, Sweis RF (2002) Acc Chem Res 35:835
Fleming I, Henning R, Plaut H (1984) Chem Commun 29:30
Tamao K, Maeda K (1986) Tetrahedron Lett 27:65
Jones GR, Landais Y (1996) Tetrahedron 52:7599
Blumenkopf TA, Overman LE (1986) Chem Rev 86:857
Kakiuchi F, Yamada A, Chatani N, Murai S, Furukawa N, Seki Y (1990) Organometallics 18:2033
Kakiuchi F, Tanaka Y, Chatani N, Murai S (1993) J Organomet Chem 456:45
Marciniec B (1992) Comprehensive handbook on hydrosilylation. Pergamon Press, Oxford
Marciniec B, Maciejewski H, Pietraszuk C, Pawluc P (2009) Hydrosilylation: a comprehensive review on recent advances. In: Marciniec B (ed) Advances in silicon science, Vol 1. Springer, Berlin, Chapters 2 and 3
Na Y, Chang S (2000) Org Lett 2:1887
Trost BM, Ball ZT (2005) J Am Chem Soc 127:17644
Trost BM, Machacek MR, Ball ZT (2003) Org Lett 5:1895
Sarah Maifeld V, Michael NT, Lee D (2005) Tetrahedron Lett 46:105
Sato A, Kinoshita H, Shinokubo H, Oshima K (2004) Org Lett 6:2217
Takeuchi R, Nitta S, Watanabe D (1996) J Org Chem 60:3045
Itami K, Mitsudo K, Nishino A, Yoshida J (2002) J Org Chem 67:2645
Wang F, Neckers DCJ (2003) J Organomet Chem 665:1
Wu W, Li CJ (2003) Chem Commun 14:1668
Bo GD, Berthon-Gelloz G, Tinant B, Markó IE (2006) Organometallics 25:1881
Hamze A, Provot O, Brion JD, Alami M (2007) Synthesis 2007:2025
Hamze A, Provot O, Brion JD, Alami M (2008) Tetrahedron Lett 49:2429
Berthon-Gelloz G, Schumers JM, Bo GD, Tinant B, Marko IE (2008) J OrgChem 73:4190
Ramírez-Oliva E, Hernández A, Martínez-Rosales JM, Aguilar-Elguezabalc A, Herrera-Pérez G, Cervantes J (2006) Arkivoc 126:136
Alonso F, Buitrago R, Moglie Y, Ruiz-Martínez J, Sepúlveda-Escribano A, Yus M (2011) J Organomet Chem 696:368
Cano R, Yus M, Ramon DJ (2012) ACS Catal 2:1070
Motoda D, Shinokubo H, Oshima K (2002) Synlett 2002:1529
Yamashita H, Uchimaru Y (1999) Chem Commun 1763
Sumida T, Kato S, Yoshida T, Hosoya (2012) Org Lett 14:1552
Kim DH, Park YW (2006) Bull Korean Chem Soc 27:27
Bandari R, Prager A,Höche T,Buchmeiser MR (2011) Arkivoc 4:54
Buchmeiser MR, Bandari R, Prager A, Lober A, Knolle W (2010) Macromol Symp 287:107
Bandari R, Buchmeiser MR (2012) Catal SciTechnol 2:220
Bandari R, Hoche T, Prager A, Dirnberger K, Buchmeiser MR (2010) Chem Eur J 16:4650
Das P, Sharma D, Shil AK, Kumari A (2011) Tetrahedron Lett 52:1176
Bandna, Aggarwal N, Das P (2011) Tetrahedron Lett 52:4954
Bandna, Guha NR, Shil AK, Sharma D, Das P (2012) Tetrahedron Lett 53:5318
Guha NR, Reddy CB, Aggarwal N, Sharma D, Shil AK, Bandna, Das P (2012) Adv Synth Catal 354:2911
Sharma D, Kumar S, Shil AK, Guha NR, Bandna, Das P (2012) Tetrahedron Lett 53:7044
Shil AK, Guha NR, Sharma D, Das P (2013) RSC Adv 3:13671
Sharma D, Reddy CB, Shil AK, Saroch RP, Das P (2013) Mol Divers 17:651
Kumar S, Das P (2013) New J Chem 37:2987
Shil AK, Das P (2013) Green Chem 15:3421
Perry RJ, Karageorgis M, Hensler J (2007) Macromolecules 40:3929
Alonso F, Buitrago R, Moglie Y, Sepulveda-Escribano A, Yus M (2012) Organometallics 31:2336
Stamos DP, Taylor AG, Kishi Y (1996) Tetrahedron Lett 37:8647
Ilardi EA, Stivala CE, Zakarian A (2008) Org Lett 10:1727
Pawluc P, Franczyk A, Walkowiak JD, Hreczycho G, Kubicki M, Marciniec B (2011) Org Lett 13:1976
Sidera M, Costa AM, Vilarrasa J (2011) Org Let 13:4934
Tsuji J (2000) Reactions of Organic Halides and Pseudohalides. Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis. Wiley, New York, pp 27–108
Hoshiya N, Isomura N, Shimoda M, Yoshikawa H, Yamashita Y, Iizuka K, Tsukamoto S, Shuto S, Arisawa M (2009) ChemCatChem 1:279
Acknowledgments
Authors are grateful to Director CSIR-IHBT for providing necessary facilities during the course of the work. We gratefully acknowledge AIRF-JNU for HRTEM analysis. The authors thank CSIR, New Delhi for financial support as part of XII Five Year Plan programme under title ORIGIN (CSC-0108). CBR (UGC-JRF), AKS (CSIR-SRF), NRG (UGC-SRF) and DS (CSIR-SRF) thanks CSIR and UGC New Delhi for awarding fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
CSIR-IHBT communication no. 3546
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bal Reddy, C., Shil, A.K., Guha, N.R. et al. Solid Supported Palladium(0) Nanoparticles: An Efficient Heterogeneous Catalyst for Regioselective Hydrosilylation of Alkynes and Suzuki Coupling of β-Arylvinyl Iodides. Catal Lett 144, 1530–1536 (2014). https://doi.org/10.1007/s10562-014-1311-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1311-8