Abstract
A series of Zn-Al composite oxides that were modified with alkaline earth metals, Zn-M-Al-O (M = Mg, Ca, Sr, and Ba) were fabricated via calcination of the corresponding hydrotalcite precursors, and evaluated as catalysts for the synthesis of propylene carbonate (PC) from CO2 and propylene oxide. Among the Zn-M-Al-O catalysts, Zn-Mg-Al-O (Zn/Mg = 4.0, pH = 10, without hydrothermal treatment) is the best in performance, showing PC selectivity of 99.2% and yield of 88.8% (140 °C, 12 h). Furthermore, the Zn-Mg-Al-O catalyst can be readily reused and recycled without any loss of activity in a test of five cycles. Through detailed studies of the basic nature of the Zn-M-Al-O catalysts, it was found that a moderate basicity (6.1 ≤ H 0 < 8.9) is beneficial to the cycloaddition reaction. The NH3- and CO2-TPD results also indicate that the Zn-Mg-Al-O catalyst has acid–base bifunctional properties, and a reaction mechanism is proposed.
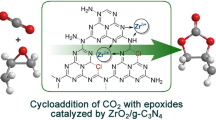
Graphic Abstract
Zn-Mg-Al composite oxides were prepared via calcination of the corresponding hydrotalcite precursors, and used as catalysts for the synthesis of propylene carbonate from CO2 and propylene oxide. We achieved high catalytic efficiency under mild conditions, easy separation of catalyst from the product, and good recyclability of the catalyst. A plausible reaction mechanism has been proposed for the catalytic action.












Similar content being viewed by others
References
Sakakura T, Choi JC, Yasuda H (2007) Chem Rev 107:2365
Sakakura T, K Kohno (2009) Chem Commun 1312
Dai WL, Luo SL, Yin SF, Au CT (2009) Appl Catal A: Gen 366:2
Lu XB, Zhang YJ, Jin K, Luo LM, Wang H (2004) J Catal 227:537
Jiang JL, Gao FX, Hua RM, Qiu XQ (2005) J Org Chem 70:381
Kim HS, Bae JY, Lee JS, Kwon OS, Jelliarko P, Lee SD, Lee SH (2005) J Catal 232:80
Ion A, Parvulescu V, Jacobs P, Vos DD (2009) Appl Catal A: Gen 363:40
Huang JW, Shi M (2003) J Org Chem 68:6705
Sun J, Wang L, Zhang SJ, Li ZX, Zhang XP, Dai WB, Mori R (2006) J Mol Catal A: Chem 256:295
Kawanami H, Ikushima Y (2000) Chem Commun 2089
Barbarini A, Maggi R, Mazzacani A, Mori G, Sartori G, Sartorio R (2003) Tetrahedron Lett 44:2931
Jiang JL, Hua RM (2006) Synth Commun 36:3141
Sun JM, Fujita SI, Zhao FY, Arai M (2004) Green Chem 6:613
Sun JM, Fujita SI, Arai M (2005) J Orgaomet Chem 690:3490 and references therein
Wu SS, Zhang XW, Dai WL, Yin SF, Li WS, Ren YQ, Au CT (2008) Appl Catal A: Gen 341:106
Sun J, Ren JY, Zhang SJ, Cheng WG (2009) Tetrahedron Lett 50:423
Bhanage BM, Fujita SI, Ikushima Y, Arai M (2001) Appl Catal A: Gen 219:259
Yano T, Matsui H, Koike T, Ishiguro H, Fujihara H, Yoshihara M, Maeshima T (1997) Chem. Commum 1129
Yamaguchi K, Ebitani K, Yoshida T, Yoshida H, Kaneda K (1999) J Am Chem Soc 121:4526
Doskocil EJ, Bordawekar SV, Kaye BG, Davis RJ (1999) J Phys Chem B 103:6277
Tu M, Davis RJ (2001) J Catal 199:85
Doskocil EJ (2004) Microporous Mesoporous Mater 76:177
Doskocil EJ (2005) J Phys Chem B 109:2315
Srivastava R, Srinivas D, Ratnasamy P (2003) Catal Lett 91:133
Fujita S, Bhanage BM, Ikushima Y, Shirai M, Torii K, Arai M (2002) Catal Lett 79:95
Lu XB, Xiu JH, He R, Jin K, Luo LM, Feng XJ (2004) Appl Catal A: Gen 275:73
Du Y, Cai F, Kong DL, He LN (2005) Green Chem 7:518
Srivastava R, Srinivas D, Ratnasamy P (2005) J Catal 233:1
Zhang XH, Zhao N, Wei W, Sun YH (2006) Catal Today 115:102
Wang JQ, Yue XD, Cai F, He LN (2007) Catal Commun 8:167
Kovanda F, Koloušek D, Cílovâ Z, Hulínsky V (2005) Appl Clay Sci 28:101
Kim TW, Sahimi M, Tsotsis T (2009) Ind Eng Chem Res 48:5794
Sankar M, Tarte NH, Manikandan P (2004) Appl Catal A: Gen 276:217
Mori K, Mitani Y, Hara T, Mizugaki T, Ebitani K, Kaneda K (2005) Chem Commun 3331
Yamanaka T, Tanabe K (1975) J Phys Chem 79:2409
Yamanaka T, Tanabe K (1976) J Phys Chem 80:1723
Oh JM, Hwang SH, Choy JH (2002) Solid State Inoics 151:285
Yasuda H, He LN, Takahashi T, Sakakura T (2006) Appl Catal A: Gen 298:177
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos 20873038 and 20507005), Outstanding Young Research Award of National Natural Science Foundation of China (Grant No E50725825), and National 863 Program of China (2009AA05Z319). CTA thanks the Hunan University for an adjunct professorship and the Hong Kong Baptist University for financial support (FRG/08-09/II-09).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dai, WL., Yin, SF., Guo, R. et al. Synthesis of Propylene Carbonate from Carbon Dioxide and Propylene Oxide Using Zn-Mg-Al Composite Oxide as High-efficiency Catalyst. Catal Lett 136, 35–44 (2010). https://doi.org/10.1007/s10562-009-0198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0198-2