Abstract
This study investigated cryopreserved pulmonary homograft (CPA) structural integrity after prolonged cold ischemic harvesting times in a juvenile sheep model. Three groups with different post-mortem cold ischemic harvesting times were studied, i.e. Group 1 (24 h, n = 10); group 2 (48 h, n = 10); group 3 (72 h, n = 10). In each group, 5 CPAs were studied in vitro after cryopreservation and thawing. The other 5 CPAs were implanted in juvenile sheep for a minimum of 180 days. Serology samples were obtained and echocardiography was performed before euthanasia. Hematoxylin and eosin (H&E), scanning electron microscopy (SEM), von Kossa, Picrosirius red, α-actin, immunohistochemistry [von Willebrand factor (vWF), CD4, CD31 and CD34] and calcium content analyses were performed on explanted CPAs. The in vitro and in vivo studies failed to demonstrate any change in tensile strength, Young’s Modulus and thermal denaturation (Td) results between the groups. SEM demonstrated a reduction in endothelial cells (50 % at 24 h, 60.9 % at 48 h and 40.9 % at 72 h), but H&E could not demonstrate autolysis in any CPA in vitro. All cultures were negative. In the explanted groups, IgE, IgM and IgG results were inconclusive. Echocardiography demonstrated normal valve function in all groups. H&E and Picrosirius red staining confirmed tissue integrity. vWF, CD31 and CD34 staining confirmed a monolayer of endothelial cells in all explanted valves. Calcium content of explanted CPA leaflets was similar. This experimental study supports the concept of prolonging the cold ischemic harvesting time of cryopreserved homografts to reduce homograft shortage.
Similar content being viewed by others
Introduction
The gold standard for reconstruction of the right ventricular outflow tract (RVOT) is cryopreserved pulmonary homografts (CPAs). Although Chambers et al. (1997) showed that freedom from reoperation following homograft reconstruction of the RVOT using the Ross procedure to be as high as 80 % at 25 years of follow-up, it is generally agreed that homografts degenerate over time. This degeneration is mostly due to immune responses (Welters et al. 2002; Neumann et al. 2014). Alternatives to homografts are xenogenic heart valves, which are available off-the-shelf. However, these valves are treated with glutaraldehyde and therefore present serious limitations for young patients due to early tissue degeneration and calcification (Holmes et al. 2012; Homann et al. 2000). A further alternative is tissue engineered pulmonary heart valves which show promising results for up to 10 years if the scaffold is a homograft (Dohmen et al. 2011; Cebotari et al. 2011). Since CPA use is limited by donor availability, especially with respect to small valve sizes, investigations are needed to increase the pool of potentially available human heart valves without compromising quality. The international norm for harvesting valves either from heart transplant recipients, beating heart donors or non-beating heart donors, is limited to 24 h post-mortem. The reason for this restraint is that increased homograft durability was demonstrated with retained cellular viability in cryopreserved homografts (Angell et al. 1989; O’Brien et al. 1995). However, Mitchell et al. (1998) argue that most homografts are acellular and that valvular performance is linked to retention of structural integrity of the valve scaffold and not cellular viability.
This study investigated CPA structural integrity after prolonged cold ischemic harvesting times in a juvenile sheep model.
Materials and methods
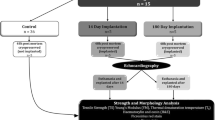
All animal experiments and surgical procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals as published by the US National Institutes of Health (NIH Publication 85-23, revised 1996). Approval of the study protocol was obtained from the Animal Ethics Committee of the University of the Free State (ETOVS nr. 12/06). A schematic presentation of the study layout is provided in Fig. 1.
Three groups with different post-mortem cold ischemic harvesting times were studied in vitro as well as in vivo, while a fourth group with valves (n = 5) harvested within 6 h post-mortem and non-cryopreserved, were used as control in the in vitro studies: group 1 (24 h, n = 10); group 2 (48 h, n = 10); group 3 (72 h, n = 10). In each of the three groups, 5 CPAs were studied in vitro after processing and thawing to evaluate tissue integrity and impact of processing. The other 5 CPAs from each group were studied in vivo through implantation in juvenile sheep, for a minimum of 180 days (group 1: ñ = 210 days; group 2: ñ = 206 days; group 3: ñ = 188 days), to evaluate valve performance, biological interaction and modes of degeneration. The population median values between the different days of implantation in the three groups proved not to differ significantly (p > 0.05) from each other. Wethers of the Dorper strain were used and each carcass and recipient animal received an ear tag with a unique identification number.
Pulmonary valve harvesting and preparation
For the control group, hearts were obtained from a local abattoir directly after slaughtering and transported on ice to the laboratory. The valves were dissected and washed in cold (4 °C) Ringers-lactate (Bodene Pty, Ltd trading as Intramed, Port Elizabeth, SA) and all required samples taken for evaluation. For the other three groups, thirty juvenile sheep were sacrificed by intravenous injection of an overdose of potassium chloride (Adcock Ingram Critical Care, Johannesburg) and pulmonary valve conduits for the preparation of cryopreserved ovine homografts were harvested at 24, 48 and 72 h respectively. Ischemic time was defined as the time between death and harvesting of the heart valve. Ischemic time was composed of a 6 h period of warm ischemia (from death to refrigeration of the cadaver in the mortuary) followed by a longer period of cold ischemia until the heart was removed from the cadaver at the designated times. The carcasses were skinned after euthanasia and the large stomach was removed to limit the effect of the ongoing intestinal fermentation process, which is typical in ruminants and would increase the body temperature and subsequently the rate of autolysis. In group 1 (n = 5) the pulmonary heart valves were removed as a valve conduit and processed after 24 h, in group 2 (n = 5) after 48 h and in group 3 (n = 5) after 72 h. The valves were rinsed, inspected for fenestrations or other abnormalities and the thickness of the myocardium was trimmed to approximately 5 mm. A competency test was performed to evaluate regurgitation control. Each valve was immersed in 100 ml of M199 with Earle’s Base (Highveld Biological (Pty) Ltd., Johannesburg, South Africa (SA) containing 2.5 mg Fungizone (Bristol-Meyers Squibb, Johannesburg, SA), 25 mg Amikacin (Fresenius, Bodene (Pty) Limited trading as Intramed, Johannesburg, SA), 50 mg Cefoxitin (Sabax, Johannesburg, SA) and 50 mg Piperacillin (Sabax, Johannesburg, SA) to sterilize for 24 h. Then the pulmonary valves were cryopreserved under sterile conditions using a Cryoson BV-9 Biological Freezer (Consarctic, Schöllkrippen, Germany). Valves were placed in a cryobag containing a solution of 100 ml M199 with 11 ml dimethylsulfoxide (DMSO) and cryopreserved at a rate of approximately −1 °C/min until −140 °C was reached. Quality control for the cryopreservation of the heart valves procured for this study was performed according to ISO 9000 standards.
Cryopreserved valves were stored in the vapor phase of liquid nitrogen (LN2) for a minimum period of 2 weeks till implantation. At thawing, the cryobags were placed on a shelf at room temperature for ±5 min to eliminate the excessive cold temperature, followed by immersion in a waterbath at 30 °C for another 5–7 min until most of the liquid inside the bag have melted. The bag was then opened sterile and the contents placed gently in two changes of 500 ml of M199 at 4 °C for 10 min each, before implantation.
In vitro study
Biomechanical testing
Biomechanical properties of the tissue samples were examined using a tensile testing apparatus (Lloyds LS100 Plus tensile strength (TS) tester, IMP, Johannesburg, SA). TS and YM were performed on tissue samples fixated with clamps at both ends and gradually stretched (0.1 mm/s) by applying constant tension on the ends (Thubrikar et al. 1983). Both pulmonary valve leaflets and wall samples underwent biomechanical testing.
Determination of Thermal Denaturation Temperature (Td) is a technique for thermal analysis, performed using differential scanning calorimetry (DSC) (Mettler Toledo, DSC 822e, Microsep, Johannesburg, SA). In DSC, the rate of heat flow to the sample is compared to the rate of heat flow to an inert material, while the materials are heated or cooled concurrently. For proteins, the thermally induced process detectable by DSC is the structural malting or unfolding of the molecule. The transition of protein from a native to a denatured conformation is accompanied by the rupture of inter- and intra-molecular bonds, and the process has to occur in a cooperative manner to be discerned by DSC (Smith and Judge 1991). This was recorded as Td for each group (Rüegg et al. 1975).
Microbiological examinations
Blood culture samples were taken intra-cardially from each intact donor heart before harvesting the heart for further dissection of the valves (groups 1–3). These samples were analyzed using standard microbiological techniques for detecting aerobic and anaerobic microbes, as well as for fungi. At cryopreservation, tissue and fluid samples from each valve were also taken for microbiological testing, and compared to the organisms cultured from blood samples taken intra-cardially at harvesting.
Histological evaluation
Specimens, for light microscopy examination, were taken from the middle of the pulmonary leaflet of each CPA as well as the wall of each CPA. These specimens were embedded in paraffin wax and two micrometer thick longitudinal sections were then prepared and routinely processed with H&E, von Kossa and Picrosirius red stainings. Specimen numbers were randomly allocated to each tissue sample, allowing completely blinded evaluation by all the evaluators for histology, immunohistochemistry and scanning electron microscopy (SEM) samples.
Immunohistochemistry
Immunohistochemical staining was performed with anti-von Willebrand factor (vWF) rabbit polyclonal antibody (Abcam, Cambridge, United Kingdom (UK), anti-CD4 rabbit polyclonal antibody (Bioss, Atlanta, United States of America (USA), anti-CD31 mouse monoclonal antibody (Abcam, Cambridge, UK), anti-CD34 goat polyclonal antibody (Santa Cruz Biotechnology Inc, Dallas, Texas, USA) and anti-α-smooth muscle actin rabbit polyclonal antibody (Abcam, Cambridge, UK). Different valvular regions were investigated, due to differing shear stresses at the leaflets compared with the rest of the valves. From each region representative samples were obtained including the inflow and outflow aspects of the valve.
Scanning electron microscopy
All valves were fixed in 2.5 % Glutaraldehyde (Merck, Johannesburg, SA). Valve leaflets were divided into two specimens of approximately 3 × 6 mm. Similar specimen samples were taken from the pulmonary wall, sinus area and pulmonary trunk. Tissue specimens were dried using the critical point method and were metalized using gold.
Evaluations were performed with a Shimadzu SSX 550 scanning electron microscope (Kyoto, Japan). The surface area of each specimen was examined and photographed at either four or five different positions, and all images were then evaluated by three independent evaluators and a score allocated. An average score for each specimen was then calculated. A three category scoring system, adapted from the six categories described by Krs et al. (2006) was used to define endothelial integrity and to evaluate the quality of the extracellular matrix (ECM) surface area. The classification of tissue was based on two key markers, endothelium and basal membrane. Category I; there is a virtual absence of both endothelium and basal membrane and the bare scaffold of the tissue is exposed. Category II; the basal membrane predominantly covers the scaffold and the endothelium is largely missing. Category III; the endothelium is virtually intact.
In vivo study
Echocardiography
Hemodynamic evaluation was performed by one experienced investigator using trans-thoracic echocardiography prior to sacrifice. A Philips Envisor Ultra Sound system (Philips, Johannesburg, SA) was used with a 3.5-MHz probe and all data were recorded. Two-dimensional trans-thoracic echocardiography was performed to evaluate morphological conditions of the valve conduit and leaflets. Additionally, the diameters of the valve annulus, sinotubular junction (ST-junction) and pulmonary artery wall were measured. Pulmonary insufficiency was evaluated semi-quantitatively using pulsed wave, continuous wave and color Doppler flow on the parasternal short axis view. The regurgitation jet across the valve was graded by identification length and width into the right ventricular outflow tract and mapped as: none/trivial, mild, moderate or severe using standard echocardiography criteria. The mean flow velocities across the implanted valves were obtained by the use of continuous wave Doppler. Each measurement was repeated six times and the mean value over the measurements was calculated. Again animals were only identified by their individual ear tag numbers, allowing blinded evaluation by the sonographer.
Serology samples
Full blood counts were analyzed on a Sysmex XE 2100 (Roche, Johannesburg, SA) according to the TF/DC detection method, hydrodynamic focusing (DC detection), flow cytometry method and a SLS-hemoglobin method. Immunoglobulins (IgG, IgA and IgM) in serum were determined quantitatively by means of immunonephelometry on a Siemens BN ProSpec Nephelometer (Siemens, Johannesburg, SA).
Gross examination
The explanted CPAs were inspected and color photographs were taken before fixation. The leaflets were inspected for fenestrations, retraction, thrombotic material, atheroma and calcification.
Calcium content analysis
Quantitative calcium analyses were performed on samples dried in a temperature controlled Scientific series 100 incubator (Lasec, Johannesburg, SA) at 45 °C for 48 h. Samples were weighed and hydrolyzed in 1 ml 50 % nitric acid and 50 µl hydrogen peroxide. Extractable calcium content was determined by inductively coupled plasma mass spectrometry Agilent ICP-MS 7500c (Chemetrix, Midrand, SA) and expressed as µg calcium per mg dry weight tissue. Only calcium content of the pulmonary leaflet and wall of the control group (<6 h) were determined pre-implantation and used as baseline values, and compared to the explanted tissues of the three ischemic groups.
Statistical analysis
For statistical analysis purposes, the cold ischemic time was described by the time effect and the source of a sample, i.e., whether the sample was sourced from the aortic leaflet or the aortic wall, was described by the source effect. Various measurements (Td, Calcium, etc.) were analyzed separately using linear models in order to determine whether there were significant time or source effects and whether there was an interaction between time and source. Time and source were treated as simple fixed effects and an interaction effect was also considered. Standard ANOVAs were also done over time and over source to obtain additional clues as to whether either effect was significant. The study was not statistically powered to detect differences due to the cost restraints involved.
Where the interaction effect in the linear model was significant, further investigation was done using ANOVA methods by separating the sources. In a few significant cases (where the time effect was significant for a specific source) the results were investigated even more deeply using Welch Two Sample t tests. This involved comparing the three timestamps in pairs. This top-down approach (starting global and drilling down) was followed throughout in order to avoid spurious results and false positives/negatives.
Where the above method was not appropriate, other methods were applied. For example, the sonar data was analyzed using Hotelling’s test and the SEM data was analyzed with Pearson’s Chi Square test. For Hotelling’s test we have to assume multivariate normality as the sample size is too small to conduct tests for this.
Surgical implantation
The juvenile sheep were male with a mean age of 4–6 months and a mean body weight of 34–40 kg. Premedication was administered with 0.175 mg/kg Neurotranq [VirbacRSA (Pty) Ltd, Halfway House, SA] and 0.2 mg/kg Atropine [Bayer (Pty) Ltd, Animal Health Division, Isando, SA] intramuscularly, and anesthesia was induced with 12 mg/kg Bomathal [Merial SA (Pty) Ltd, Halfway House, SA] intravenously. The sheep were intubated, ventilated and positioned in a lateral decubitus position. Arterial cannulation for cardiopulmonary bypass was obtained via the left carotid artery. The stump pressure of the tied-off distal carotid artery was used for invasive arterial pressure measurement. A left mini-thoracotomy was performed and the fourth rib removed. The pulmonary artery was transected and the native pulmonary valve leaflets were excised. The CPAs, with a diameter of ±16 mm, were implanted as an interposition, with two continuous 4/0 polypropylene suture anastomoses. The sheep was weaned from cardiopulmonary bypass, the mini-thoracotomy was closed in layers and a chest drain was inserted. Systemic pain medication, in the form of 2 mg Morphine sulphate [Bodene (Pty) Ltd, trading as Intramed, Port Elizabeth, SA] intramuscularly twice a day and 5 mg Depomycin [INTERVET SA (Pty) Ltd, Johannesburg, SA] daily as antibiotic, was administered for 5 days post-operatively. Animals were extubated 2–4 h post-operatively. Underwater drains and pressure lines were removed before animals were transferred to an overnight facility.
Results
In vitro study
Biomechanical testing
The following baseline mechanical results were obtained in pulmonary homografts harvested within 6 h after death. These samples were obtained from valves of juvenile sheep from a local abattoir. The mean TS were 1.04 ± 0.36 MPa for the pulmonary wall and 1.24 ± 0.79 MPa for the pulmonary leaflet. The mean TS for the pulmonary wall in the three ischemic groups were as follows: (group 1: 1.03 ± 0.36 MPa; group 2: 0.95 ± 0.22 MPa; group 3: 1.16 ± 0.34 MPa). The mean TS for the pulmonary leaflets in the three ischemic groups were as follows: (group 1: 2.57 ± 1.07 MPa; group 2: 2.93 ± 0.50 MPa; group 3: 3.03 ± 1.02 MPa).
The mean YM baseline value was 2.06 ± 0.77 MPa for the pulmonary wall and 5.68 ± 4.57 MPa for the pulmonary leaflet. The mean YM for the pulmonary wall in the three ischemic groups were as follows: (group 1: 3.21 ± 0.90 MPa; group 2: 3.26 ± 0.93 MPa; group 3: 3.81 ± 1.31 MPa). The mean YM for the pulmonary leaflets in the three ischemic groups were as follows: (group 1: 9.93 ± 2.63 MPa; group 2: 9.25 ± 5.02 MPa; group 3: 10.73 ± 4.33 MPa).
The mean Td baseline values were 70.57 ± 1.03 °C for the pulmonary wall and 67.16 ± 1.31 °C for the pulmonary leaflets. The mean Td for the pulmonary wall in the three ischemic groups were as follows: (group 1: 70.57 ± 1.12 °C; group 2: 71.19 ± 1.13 °C; group 3: 72.44 ± 2.85 °C). The mean Td for the pulmonary leaflets in the three ischemic groups were as follows: (group 1: 70.25 ± 0.76 °C; group 2: 71.25 ± 1.17 °C; group 3: 71.01 ± 0.36 °C).
In vitro evaluation of the TS, YM and Td temperatures did not reveal any statistically significant differences (p > 0.05) between the 24, 48 and 72 h groups.
Microbiological examinations
Microbiological examination detected that the initial microbes present on the CPAs in the different groups were as follows: group 1 (n = 5): no aerobic organisms and 3 positive cultures for anaerobic microorganisms; group 2 (n = 5): 2 positive samples for aerobic organisms and 1 positive sample for anaerobic organism; group 3 (n = 5): 3 samples were positive for aerobic microorganisms and 3 samples were positive for anaerobic microorganisms. The most common microorganisms were gram + bacilli and coagulase negative staphylococci. After 24 h of sterilization in the nutrient antibiotic solution no organisms could be cultured in the pre-cryopreserved specimens. No fungal cultures were positive at any point in time.
Histological evaluation
The histological findings in the control group (harvested <6 h post-mortem) are described as reference.
H&E staining showed endothelial-like cells on the surface of the control valves; however, a confluent layer was not present in all of the specimens. The ECM exhibited a normal configuration with an absence of abnormalities in the collagen and elastin structures, and this was confirmed with Picrosirius red staining. The spindleform-shaped fibroblast-like cells were arranged in a parallel layout; however, the number of cells in some of the samples was limited. Endothelial-like cells were confirmed to be endothelial cells by positive expression of vWF and CD31. Interstitial cells were confirmed to be myofibroblasts since these cells were α-actin positive. No differences between the groups were observed post processing and thawing, compared to the control group.
Scanning electron microscopy
According to the modified Krs et al. (2006) and Smit, PhD, unpublished data, 2011 classification, the baseline samples of the pulmonary leaflets were all in category III (100 %) and, for the pulmonary wall samples, 86.7 % were in category II and 13.3 % were in category I. In group 1 (24 h), 12.5 % of the pulmonary leaflets fell in category I, 37.5 % in category II and 50 % in category III, while 96 % of the pulmonary wall samples fell in category I and 4 % in category II. In group 2 (48 h), 21.7 % of the pulmonary leaflets fell in category I, 60.9 % in category II and 17.4 % in category III, while 100 % of the pulmonary wall samples fell in category I. In group 3 (72 h), 18.2 % of the pulmonary leaflets fell in category I, 40.9 % in category II and 40.9 % in category III, while 100 % of the pulmonary wall samples fell in category I.
No statistically significant differences (p > 0.05) in SEM was found between any of the three ischemic groups.
Calcium content analysis
The mean baseline quantitative calcium content was 1.8 ± 0.09 µg/mg of dry weight for the pulmonary leaflet samples and 0.80 ± 0.06 µg/mg of dry weight for the pulmonary wall samples. Only the baseline values of the control group (<6 h) were determined and compared to the explanted tissues in the three ischemic groups.
In vivo study
Echocardiography
Echocardiographic examinations showed limited morphological calcification. The calcification was in the range of none to mild for the valve leaflets in all the groups. Mild to moderate calcification was observed in the valve annulus and in the pulmonary wall. There were however no differences between the groups.
The mean pressure gradients at the CPAs within the different groups were 12.0 ± 5.6 mmHg in group 1, 10.7 ± 3.6 mmHg in group 2 and 10.5 ± 2.6 mmHg in group 3 respectively. There were no statistically significant differences between the groups (group 1 vs 2, p = 0.70; group 1 vs 3, p = 0.61; group 2 vs 3, p = 0.94). The mean annulus diameter in group 1 was 20.2 ± 2.2 mm, in group 2 it was 20.4 ± 2.6 mm, and in group 3 it was 20.5 ± 1.5 mm. The mean diameter at the ST-junction was 18.4 ± 1.4 mm in group 1, 19.3 ± 1.5 mm in group 2 and 20.1 ± 2.1 mm in group 3. The mean diameter of the pulmonary artery was 20.2 ± 1.5 mm in group 1, 20.5 ± 1.3 mm in group 2 and 20.5 ± 1.4 mm in group 3.
Serology samples
The mean total IgG for group 1 was 7.5 ± 8.9 kU/l, for group 2, 7.3 ± 10.9 kU/l and for group 3, 37.0 ± 31.1 kU/l. The mean IgA and IgM values were below 0.25 g/l for all three groups. There was no obvious trend that would identify a marked immunological process in any specific group.
Gross examination
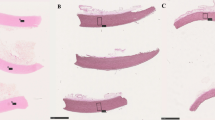
There was an absence of aneurysm formation, deformations and thrombi in all of the explanted CPAs from the three groups (Fig. 2). The leaflets were pliable without fenestrations in all the samples from the three groups (Fig. 3).
Biomechanical testing
The TS, YM and Td of the leaflets and wall specimens are summarized in Table 1. No statistically significant differences were found between groups, nor did they differ from the baseline results. Certainly no loss of strength could be demonstrated in any of the groups compared to the baseline values.
Histological evaluation
The histological examinations are presented in Fig. 4a–c. In the H&E staining results there was evidence that all samples from group 1 were covered by a monolayer of endothelial-like cells. Acellularity of the ECM in group 1 was between 30 and 90 %. Eighty percent of the CPAs in group 2 were covered by endothelial-like cells. The ECM was completely acellular in 2 samples. The other CPAs in group 2 were between 0 and 90 % acellular. In group 3, all of the CPAs were covered by a monolayer of endothelial-like cells. Acellularity was found in one valve, whereas the other valves were between 20 and 80 % acellular. von Kossa staining detected severe calcification of a leaflet in one of the explanted CPAs from group 3. None of the other explanted CPAs showed calcification of the leaflets. The pulmonary wall of only one of the CPAs from group 1 showed mild calcification. In group 2, there was severe wall calcification in two of the CPAs, moderate calcification in two CPAs and no calcification in one of the CPAs. Moderate calcification was observed in the wall of one of the explanted CPAs from group 3. None of the other explanted CPAs in group 3 exhibited calcification. Picrosirius red staining showed a well preserved ECM in all samples from the three groups. Endothelial–like cells covering the valve surfaces were identified as such since they were vWF, CD31 and CD34 positive. Interstitial cells were α-actin positive, however, since no CD4 positive cells were found, none of the samples showed inflammatory cells within the ECM.
Histological findings of explanted CPAs. a group 1 (24 h), b group 2 (48 h), and c group 3 (72 h). For each group; 1 H&E staining, 2 Picrosirius red staining, 3 α-actin staining, 4 vWF staining, 5 CD4 staining, 6 CD31 staining and 7 SEM. [h hours, H&E Hematoxylin and eosin, vWF von Willebrand factor, SEM scanning electron microscopy]
Scanning electron microscopy
After explantation, SEM was able to demonstrate that 88.0 % of the pulmonary leaflets from group 1 fell in category III and 12.0 % fell in category II. In group 2, 62.7 % of the leaflets were classified as category III, 20.0 % as category II and 17.3 % as category I. In group 3, 64.0 % of the leaflets fulfilled the criteria for category III, 12.0 % for category II and 24.0 % for category I.
In group 1, 37.3 % of the CPA pulmonary wall specimens were classified as category III, 37.3 % as category II, and 25.4 % as category I. In group 2, 26.7 % of the pulmonary wall specimens fulfilled the criteria for category III, 37.3 % for category II and 36.0 % for category I. Finally, group 3 had 12.0 % of pulmonary wall specimens in category III, 61.3 % in category II and 26.7 % in category I.
Analysis of the SEM data for both the pulmonary leaflet and wall showed a time-related change over the categories (p < 0.005 respectively). Although a gradual deterioration in the endothelial covering of both the pulmonary leaflets and walls in relation to the ischemic time was observed, no differences in structural integrity could be demonstrated.
Calcium content analysis
Mean quantitative calcium content in the pulmonary leaflets was 0.18 ± 0.13 µg/mg dry weight in group 1, 0.30 ± 0.57 µg/mg dry weight in group 2 and 0.05 ± 0.05 µg/mg dry weight in group 3. There is no difference between calcification in the explanted pulmonary leaflets between the three groups (p = 0.518).
For the pulmonary wall the mean quantitative calcium content was 16.12 ± 24.40 µg/mg dry weight in group 1, 84.95 ± 16.07 µg/mg dry weight in group 2 and 68.11 ± 41.64 µg/mg dry weight in group 3. The pulmonary wall calcification does not differ between the groups (p = 0.218). Note that only one pulmonary wall had significantly elevated calcium content.
Discussion
The pulmonary homograft remains the gold standard for right ventricle outflow tract (RVOT) reconstruction; its use is however limited by supply. The worldwide practice of limiting homograft harvesting to beating heart donors, or within 24 h post-mortem, may contribute to this shortage. If post-mortem harvesting times can be extended safely, thereby allowing more time to obtain consent or fulfill other legal requirements, cadaver-based donor programs can be expanded or in some cases be re-opened. This depends on whether viability of homografts contributes significantly to attenuate homograft failure as claimed by O’Brien et al. (1991). On the other hand Mitchell et al. (1998) considers all homografts to be eventually acellular and thus non-viable. It is thus possible to view homografts as biological scaffolds.
If all homografts eventually become acellular and function as a biological scaffold, for the purpose of this experimental animal study, it was important to firstly evaluate the impact of extending ischemic harvesting times on the morphology, structural and strength characteristics of homografts, and taking the historical perspectives on homograft biology and degeneration into consideration.
Secondly, valve function and outcomes of the biological interactions post implantation were evaluated in vivo in a standard juvenile ovine model.
Long-term durability of CPAs has previously been correlated with warm- and cold ischemic times. Cell metabolism is unchanged if the cold ischemic time is <24 h and the warm ischemic time is <12 h (Lang et al. 1994; Hu et al. 1989). O’Brien et al. (1991) stated that long-term durability is dependent on the viability of a homograft and therefore the CPA needed to be prepared within 24 h post-mortem. There is, however, no definition of preferred warm and cold ischemic times. In the literature, different homograft banks have customized protocols for processing and cryopreservation of homografts. There are also variations in the ischemic time between harvesting and cryopreservation.
Several studies have addressed homograft viability. Yankah and Hetzer (1987) reported that only 24 % of the endothelial cells survive after 2 h of exposure to room temperature. Crescenzo et al. (1992) noted the relationship between ischemic time and progression of fibroblast cell damage, which is reversible with a warm ischemic time of up to 12 h. However, a warm ischemic time longer than 12 h will lead to apoptosis. Fibroblast response to warm ischemic time is correlated with morphometric measurements (St Louis et al. 1991; Brockbank and Bank 1987).
Significant decreases in freedom of reoperation in the cryopreserved homografts compared to the 4 °C stored homografts were observed in medium term studies. However, in a recent single-center study including 1,022 patients, O’Brien et al. (2001) compared 4 °C stored homografts with viable cryopreserved homografts. In this 29-year follow-up study the viability of homografts progressively reduced, resulting in non-viability after a few days, regardless of whether the homografts were cryopreserved or stored in the refrigerator, and whether they were stored in a balanced salt solution or a nutrient medium. Thus the viable homograft theory did not survive the test of time.
Barrat-Boyes et al. (1987) conducted a long-term follow-up study including 248 patients in which valve incompetence was assessed. Significant freedom of incompetence of antibiotic-sterilized aortic homografts was found in 95 % after 5 years, 78 % after 10 years and 42 % at 14 years. Harvesting time was <24 h in 147 donors, between 24 and 48 h in 88 donors and between 49 and 75 h in 7 donors. Harvesting time was not recorded for 43 of the donors. A multivariate analysis showed that advanced donor valve age ≥55 years (CI 1.12 ± 0.30; p = 0.0002), aortic root size >30 mm (CI 1.39 ± 0.41; p = 0.0007), and recipient age <15 years (CI 2.03 ± 0.61; p = 0.0008) are independent risk factors for homograft incompetence.
Langley et al. (1996) reported, based on a series of 249 patients, a freedom of reoperation of sub-coronary implanted antibiotic sterilized valves with a warm ischemic time of 24 h and cold ischemic time of a maximum of 3 months. The freedom of reoperation of the aortic valve was excellent with 49.7 ± 5.6 % at 20 years follow-up.
The mean warm ischemic time of homografts prepared at the European Homograft Bank is <6 h. However, due to post-mortem delay, the average cold ischemic harvesting time is 24 h, up to a maximum of 36 h (Goffin et al. 1996). The clinical data of these homografts, presented by Meyns et al. (2005) shows that the ischemic time has no statistical influence on the long-term durability of these homografts when implanted into pediatric patients. Only non-anatomic position (p = 0.001), smaller graft size (p < 0.0001), younger age (on square root scale, p < 0.0001) and clamp time (p = 0.01) remain as independent risk factors.
On the other hand, Tweddell et al. (2000) investigated the longevity of homografts used to reconstruct the RVOT in 205 patients with congenital heart disease and a mean age of 6.9 ± 7.6 years (range 3 days to 48 years). Freedom from homograft failure was 54 ± 7 % at 10 years follow-up. They found that independent risk factors for homograft failure in a multivariate analysis were younger age (p < 0.001), longer warm ischemic time (p < 0.001), Z-value <2 (p = 0.03), and aortic homograft (p = 0.04).
Importantly, Kadoba et al. (1991) performed a study of cryopreserved aortic homografts in a lamb model in which the cold ischemic time was extended up to 48 h. The 48 h group performed as well as the fresh and 24 h groups. The authors concluded that expanding the pool for homografts is feasible by increasing the cold ischemic time. However, no specific tests or histological examinations were performed to systematically evaluate the allograft scaffolds or the quality of the valvular tissue as in the present study.
In the present in vitro study, we could demonstrate the presence of endothelial cells in all groups. However, no pattern was observed between the presence of endothelial cells and cold ischemic harvesting times. These findings suggest that the number of endothelial and interstitial cells present in the 72 h group were comparable with the number of cells in the 24 and 48 h groups. Thus, if the presence of endothelial cells was used to assess suitability, there should be no limitation on increasing the cold ischemic harvesting time. No testing was performed to compare the viability of the different cell types in this study as all samples were harvested after 24 h ischemic time. This 24 h period was conclusively demonstrated in previous studies to be associated with non-viability (Arminger 1995).
The in vivo part of this study showed that implanted CPAs, from the different cold ischemic harvesting time groups, exhibited intra-luminal coverage with endothelial cells. These endothelial cells are most probably of host origin since, at implantation, most of the endothelial cells were either no longer present or non-viable after at least 24 h post-mortem. However, additional investigation will be required to confirm the origin of these endothelial cells. Gradual loss of endothelial cells with prolonged ischemic times might not be detrimental to the clinical durability of the homograft, as the immune response of the recipient to the implant is severely diminished.
Previous studies have revealed that re-endothelialization is commonly seen in the juvenile sheep model (Dohmen et al. 2006a, b). This can however not be generalized to humans, since this process seems to be more restricted and takes longer in humans (Dohmen et al. 2007; Konertz et al. 2011). Further investigation is needed to better understand the recellularization of decellularized heart valves implanted into patients. The endothelial cells also need to be investigated for their functionality, as they have a major determinant function. Endothelium normally inhibits thrombus formation and leukocyte adhesion, regulates vasomotor function, and inhibits smooth muscle cell proliferation. If there is damage to the endothelium interaction with inflammatory cells and interstitial cells, by expression of vascular adhesion, molecules such as VCAM-1, ICAM-1 and ELAM-1 will change (Ardehali et al. 1995). The intercellular network via macrophages, T-lymphocytes, endothelial cells and smooth muscle cells is generated by a variety of stimulatory cytokines (IL-1, IL-2, IL-6, and tumor necrosis factor-α) and growth factors (PDGF, IGF-1, FGF, HB-EGF, EGF, GM-CSF, and TGF-β) (Duquesnoy and Demetris 1995). Therefore, it is of great interest that autologous endothelial cells overgrow the CPAs to restore the function of the interstitial cells and decrease the activation of the inflammatory cells. On the contrary, it is important to avoid a pseudo-intima formation on the leaflet surface since this will lead to leaflet retraction, resulting in central valve regurgitation (Affonso da Costa et al. 2004). Pseudo-intima formation was not observed in any of the implanted valves in this study.
A well-known disadvantage of viable CPAs in transplant surgery is that donor endothelial and interstitial cells evoke an immune response from the host (Jane-wit et al. 2013). Decellularization of a CPA could attenuate the humoral immune response to donor HLA after implantation of a CPA as was shown by Kneib et al. (2012). Homografts with viable endothelium and interstitial cells have a significantly higher number of immunogenic epitopes, for HLA classes I and II, than the decellularized groups exhibit, and should therefore initiate a more dramatic immune response from the recipient. In this study, with harvesting times in excess of 24 h, endothelial viability is highly unlikely.
On a cellular level there were no differences between the groups with varying cold ischemic times. The extracellular matrix was tested to evaluate if the strength of the scaffold would change by extending the cold ischemic harvesting time. No differences in strength of the valve tissue were detected. Furthermore, there was no increase in tissue denaturation, evaluated by measuring the Td, in any of the groups. The extracellular matrix was also confirmed to be intact by histological examination. From the in vitro data we are able to conclude that tissue strength is unaltered and therefore extending the cold ischemic harvesting time is possible.
From the in vivo investigations, with a minimum follow-up of 150 days in a juvenile sheep model, hemodynamic data shows no differences between the groups when the cold ischemic time was increased. Furthermore, the histological examinations showed a normal intact extracellular matrix. The immunohistochemistry results in this study did not show any differences in inflammatory reactions when cold ischemic time was increased. An overgrowth of endothelial cells was observed in all groups. Therefore, in this study, there were no contra-indications to extending the cold ischemic harvesting time in order to increase the number of viable homografts.
Limitations
The limitations of this study are that data provided by the juvenile sheep model cannot be unconditionally applied to human patients, since re-endothelialization is more extensive in this model than in humans. Also, the functionality and viability of the endothelial and interstitial cells were not investigated in this study.
Conclusions
This experimental study supports the concept that, with a limited warm ischemic time, the cold ischemic harvesting time of cryopreserved homografts can be prolonged. This could be a way to reduce homograft shortage since by increasing time limits for harvesting, the opportunities for obtaining consent and facilitating cadaver donor programs are increased. However, long-term clinical hemodynamic evaluation is needed to confirm this approach.
References
Angell WW, Oury JH, Lamberti JJ, Koziol J (1989) Durability of the viable aortic homograft. J Thorac Cardiovasc Surg 98:48–55
Ardehali A, Laks H, Drinkwater DC, Ziv E, Drake TA (1995) Vascular cell adhesion molecule-1 is induced on vascular endothelia and medial smooth muscle cells in experimental cardiac homograft vasculopathy. Circulation 92:450–456
Arminger LC (1995) Viability studies of human valves prepared for use as allografts. Ann Thorac Surg 60:S118–S121
Barrat-Boyes BG, Roche AHG, Subramanyan R, Pemberton JR, Whotlock RML (1987) Long-term follow-up of patients with the antibiotic-sterilized aortic homograft valve inserted freehand in the aortic position. Circulation 75:768–777
Brockbank KG, Bank HL (1987) Measurement of post cryopreservation viability. J Card Surg 2:S145–S151
Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S (2011) Use of fresh decellularized homografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 124(11 Suppl):S115–S123
Chambers JC, Somerville J, Stone S, Ross DN (1997) Pulmonary autograft procedure for aortic valve disease: long-term results of the pioneer series. Circulation 96:2206–2214
Crescenzo DG, Hilbert SL, Barrick MK, Corcoran PC, St Louis JD, Messier RH, Ferrans VJ, Wallace RB, Hopkins RA (1992) Donor heart valves: electron microscopic and morphometric assessment of cellular injury induced by warm ischemia. J Thorac Cardiovasc Surg 103:253–257
da Costa FDA, Dohmen PM, Lopes SV, Lacerda G, Pohl F, Vilani R, Da Costa MBA, Vieira ED, Yoschi S, Konertz W, da Costa IA (2004) Comparison of cryopreserved homografts and decellularized porcine heterografts implanted in sheep. Artif Organs 28:366–370
Dohmen PM, da Costa F, Yoshi S, Lopes SV, da Souza FP, Vilani R, Wouk AF, da Costa M, Konertz W (2006a) Histological evaluation of tissue-engineered heart valves implanted in the juvenile sheep model: is there a need for in vitro seeding? J Heart Valve Dis 15:823–829
Dohmen PM, da Costa F, Holinski S, Lopes SV, Yoshi S, Reichert LH, Villani R, Posner S, Konertz W (2006b) Is there a possibility for a glutaraldehyde-free porcine heart valve to grow? Eur Surg Res 38:54–61
Dohmen PM, Lembcke A, Holinski S, Kivelitz D, Braun JP, Pruss A, Konertz W (2007) Mid-term clinical results using a tissue-engineered pulmonary valve to reconstruct the right ventricular outflow tract during the Ross procedure. Ann Thorac Surg 84:729–736
Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W (2011) Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg 92:1308–1314
Duquesnoy RJ, Demetris AJ (1995) Immunopathology of cardiac transplant rejection. Curr Opin Cardiol 10:193–206
Goffin Y, Grandmougin D, Van Hoeck B (1996) Banking cryopreserved heart valves in Europe: assessment of a 5-year operation in an international tissue bank in Brussels. Eur J Cardiothorac Surg 10:505–512
Holmes AA, Co S, Human DG, Leblanc JG, Campbell AI (2012) The Contegra conduit: late outcomes in right ventricular outflow tract reconstruction. Ann Pediatr Cardiol 5:27–33
Homann M, Haehnel JC, Mendler N, Paek SU, Holper K, Meisner H, Lange R (2000) Reconstruction of the RVOT with valved biological conduits: 25 years experience with homografts and xenografts. Eur J Cardiothorac Surg 17:624–630
Hu JF, Gilmer L, Hopkins R, Wolfinbarger L Jr (1989) Effects of antibiotics on cellular viability in porcine heart valve tissue. Cardiovasc Res 23:960–964
Jane-wit D, Manes TD, Yi T, Qin L, Clark P, Kirkiles-Smith NC, Abrahimi P, Devalliere J, Moeckel G, Kulkarni S, Tellides G, Pober JS (2013) Alloantibody and complement promote T cell-mediated cardiac homograft vasculopathy through noncanonical nuclear factor-κB signaling in endothelial cells. Circulation 128:2504–2516
Kadoba K, Armiger L, Sawatari K, Jonas RA (1991) Influence of time from donor death to graft harvest on conduit function of cryopreserved aortic homografts in lambs. Circulation 84(Suppl. 5):III100–III111
Kneib C, von Glehn CQ, Costa FD, Costa MT, Susin MF (2012) Evaluation of humoral immune response to donor HLA after implantation of cellularized versus decellularized human heart valve homografts. Tissue Antigens 80:165–174
Konertz W, Angeli E, Tarusinov G, Christ T, Kroll J, Dohmen PM, Krogmann O, Franzbach B, Napoleone CP, Gargiulo G (2011) Right ventricular outflow tract reconstruction with decellularized porcine xenografts in patients with congenital heart disease. J Heart Valve Dis 20:341–347
Krs O, Burkert J, Slízová D, Kobylka P, Spatenka J (2006) Homograft semilunar cardiac valves processing and cryopreservation—morphology in scanning electron microscope. Cell Tissue Bank 7:167–173
Lang SJ, Giordance MS, Cardon-Cardo C, Summers BD, Staino-Cioco L, Hajjar DP (1994) Biochemical and cellular characterization of cardiac valve tissue after cryopreservation or antibiotic preservation. J Thorac Cardiovasc Surg 108:63–67
Langley SM, Livesey SA, Tsang VT, Barron DJ, Lamb RK, Ross JK, Monro JL (1996) Long-term results of valve replacement using antibiotic-sterilized homografts in the aortic position. Eur J Cardiothorac Surg 10:1097–1106
Meyns B, Jashari R, Gewillig M, Mertens L, Komarek A, Lesaffre E, Budts W, Daenen W (2005) Factors influencing the survival of cryopreserved homografts. The second homograft performs as well as the first. Eur J Cardiothorac Surg 28:211–216
Mitchell RN, Jonas RA, Schoen FJ (1998) Pathology of explanted cryopreserved allograft heart valves: comparison with aortic valves from orthotopic heart transplants. J Thorac Cardiovasc Surg 115:118–127
Neumann A, Sarikouch S, Breymann T, Cebotari S, Boethig D, Horke A, Beerbaum P, Westhoff-Bleck M, Harald B, Ono M, Tudorache I, Haverich A, Beutel G (2014) Early systemic cellular immune response in children and young adults receiving decellularized fresh homografts for pulmonary valve replacement. Tissue Eng Part A 20:1003–1011
O’Brien MF, Harrocks S, Stafford EG, Gardner MA, Pohlner PG, Tesar PJ, Stephens F (2001) The homograft aortic valve: a 29-year, 99.3 % follow-up of 1,022 valve replacements. J Heart Valve Dis 10:334–344
O’Brien MF, McGiffin DC, Stafford EG, Gardner MA, Pohlner PF, McLachlan GJ, Gall K, Smith S, Murphy E (1991) Homograft aortic valve replacement: long-term comparative clinical analysis of the viable cryopreserved and antibiotic 4 degrees C stored valves. J Card Surg 6(Suppl 4):S534–S543
O’Brien MF, Stafford EG, Gardner MA (1995) Homograft aortic valve replacement: long-term follow-up. Ann Thorac Surg 60:S65–S70
Rüegg M, Moor U, Blanc B (1975) Hydration and thermal denaturation of beta-lactoglobulin: a calorimetric study. Biochim Biophys Acta 400:334–342
Smith SH, Judge MD (1991) Relationship between pyridinoline concentration and thermal stability of bovine intramuscular collagen. J Anim Sci 69:1989–1993
St Louis J, Corcoran P, Rajan S, Conte J, Wolfinbarger L, Hu J, Lange PL, Wang YN, Hilbert SL, Analouei A (1991) Effects of warm ischemia following harvesting of homograft cardiac valves. Eur J Cardiothorac Surg 5:458–464
Thubrikar MJ, Deck JD, Aouad J, Nolan SP (1983) Role of mechanical stress in calcification of aortic bioprosthetic valves. J Thorac Cardiovasc Surg 86:115–125
Tweddell JS, Pelech AN, Frommelt PC, Mussatto KA, Wyman JD, Fedderly RT, Berger S, Frommelt MA, Lewis DA, Friedberg DZ, Thomas JP Jr, Sachdeva R, Litwin SB (2000) Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation 102(Suppl. 3):III130–III135
Welters MJ, Oei FB, Witvliet MD, Vaessen LM, Cromme-Dijkhuis AH, Bogers AJ, Weimar W, Claas FH (2002) A broad and strong humoral immune response to donor HLA after implantation of cryopreserved human heart valve homografts. Hum Immunol 63:1019–1025
Yankah AC, Hetzer R (1987) Procurement and viability of cardiac valve homografts. In: Yankah AC (ed) Cardiac valve homografts 1962–1987. Springer, Berlin, pp 23–26
Conflict of interest
We certify that all authors named in this manuscript deserve authorship, and that all authors have agreed to be listed and have read and approved the manuscript and its submission to Cell and Tissue Banking. The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smit, F.E., Bester, D., van den Heever, J.J. et al. Does prolonged post-mortem cold ischemic harvesting time influence cryopreserved pulmonary homograft tissue integrity?. Cell Tissue Bank 16, 531–544 (2015). https://doi.org/10.1007/s10561-015-9500-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-015-9500-2