Abstract
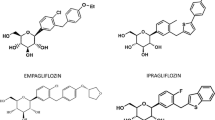
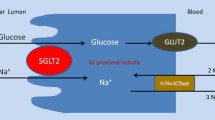
Type 2 diabetes is characterized by decreased insulin secretion and sensitivity. The available oral anti-diabetic drugs act on many different molecular sites. The most used of oral anti-diabetic agents is metformin that activates glucose transport vesicles to the cell surface. Others are: the sulphonylureas; agents acting on the incretin system; GLP-1 agonists; dipetidylpeptidase-4 inhibitors; meglinitide analogues; and the thiazolidinediones. Despite these many drugs acting by different mechanisms, glycaemic control often remains elusive. None of these drugs have a primary renal mechanism of action on the kidneys, where almost all glucose excreted is normally reabsorbed. That is where the inhibitors of glucose reuptake (sodium-glucose cotransporter 2, SGLT2) have a unique site of action. Promotion of urinary loss of glucose by SGLT2 inhibitors embodies a new principle of control in type 2 diabetes that has several advantages with some urogenital side-effects, both of which are evaluated in this review. Specific approvals include use as monotherapy, when diet and exercise alone do not provide adequate glycaemic control in patients for whom the use of metformin is considered inappropriate due to intolerance or contraindications, or as add-on therapy with other anti-hyperglycaemic medicinal products including insulin, when these together with diet and exercise, do not provide adequate glycemic control. The basic mechanisms are improved β-cell function and insulin sensitivity. When compared with sulphonylureas or other oral antidiabetic agents, SGLT2 inhibitors provide greater HbA1c reduction. Urogenital side-effects related to the enhanced glycosuria can be troublesome, yet seldom lead to discontinuation. On this background, studies are analysed that compare SGLT2 inhibitors with other oral antidiabetic agents. Their unique mode of action, unloading the excess glycaemic load, contrasts with other oral agents that all act to counter the effects of diabetic hyperglycaemia.

Similar content being viewed by others
References
Opie LH, Yellon DM, Gersh BJ. Controversies in the cardiovascular management of type 2 diabetes. Heart. 2011;97:6–14.
Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;11, CD008143.
Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–13.
Lee YK, Song SO, Kim KJ, et al. Glycemic effectiveness of metformin-based dual-combination therapies with sulphonylurea, pioglitazone, or DPP4-Inhibitor in drug-naïve Korean type 2 diabetic patients. Diabetes Metab J. 2013;37:465–74.
Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56.
Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–43.
Richard KR, Shelburne JS, Kirk JK. Tolerability of dipeptidyl peptidase-4 inhibitors: a review. Clin Ther. 2011;33:1609–29.
Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vasc Pharmacol. 2011;55:10–6.
Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2.. Am J Physiol Cell Physiol. 2012 Aug 1. PMID 22673616.
Lin JT, Szwarc K, Kinne R, Jung CY. Structural state of the Na+/D-glucose cotransporter in calf kidney brush-border membranes. Target size analysis of Na+-dependent phlorizin binding and Na+-dependent D-glucose transport. Biochim Biophys Acta. 1984;777:201–8.
Adachi T, Yasuda K, Okamoto Y, et al. T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats. Metabolism. 2000;49:990–5.
Katsuno K, Fujimori Y, Takemura Y, et al. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. Pharmacol Exp Ther. 2007;320:323–30.
Rydén L, Grant PJ. ESC Pocket Guidelines, version 2013. Committee for Practice Guidelines. European Association for the Study of Diabetes and European Society of Cardiology. Guidelines on diabetes, pre-diabetes and cardiovascular diseases, 2013, page 17.www.escardioi.org/guidelines.
Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035–87.
Carlson GF, Tou CK, Parikh S, Birmingham BK, Butler K. Evaluation of the effect of dapagliflozin on cardiac repolarization: a thorough QT/QTc study. Diabetes Ther. 2011;2:123–32.
Zhang W, Smulders R, Abeyratne A, et al. Ipragliflozin does not prolong QTc interval in healthy male and female subjects: a phase I study. Clin Ther. 2013;35:1150–61.
Smulders RA, Zhang W, Veltkamp SA, et al. No pharmacokinetic interaction between ipragliflozin and sitagliptin, pioglitazone, or glimepiride in healthy subjects. Diabetes Obes Metab. 2012;14:937–43.
Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82.
Goring S, Hawkins N, Wygant G, et al. Dapagliflozin compared with other oral anti-diabetes treatments when added to metformin monotherapy: a systematic review and network meta-analysis. Diabetes Obes Metab. 2013 Nov 14. doi: 10.1111/dom.12239. [Epub ahead of print].
Ji L, Ma J, Li H, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. 2014;36:84–100.
Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74.
Johnsson KM, Ptaszynska A, Schmitz B, et al. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complicat. 2013;27:473–8.
Kasichayanula S, Chang M, Liu X, et al. Lack of pharmacokinetic interactions between dapagliflozin and simvastatin, valsartan, warfarin, or digoxin. Adv Ther. 2012;29:163–7712.
Reilly TP, Graziano MJ, Janovitz EB, et al. Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an Inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014 Jan 29. [Epub ahead of print].
Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013 May;125(3):181–9.
Chan HW, Ashan B, Jayasekera P, Collier A, Ghosh S. A new class of drug for the management of type 2 diabetes: sodium glucose co-transporter inhibitors: ‘glucuretics’. Diabetes Metab Syndr. 2012;6:224–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Opie, L.H. Sodium Glucose Co-transporter 2 (SGLT2) Inhibitors: New among Antidiabetic Drugs. Cardiovasc Drugs Ther 28, 331–334 (2014). https://doi.org/10.1007/s10557-014-6522-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-014-6522-0