Abstract
Purpose
In this study, the effect of heparin-derived oligosaccharide (HDO) on vascular endothelial growth factor (VEGF) induced vascular smooth muscle cell (VSMC) proliferation and the signal transduction mechanisms involved were investigated.
Methods
MTT assays were used to measure VSMC proliferation, flow cytometry to analyze cell cycle distribution, RT-PCR for detection of gene transcript levels, and cell-based ELISA, Western blotting and immunocytochemical methods to detect the expression of PKC-α, ERK 1/2, p-ERK 1/2, Akt, p-Akt, p-PDK1 and p-GSK-3β.
Results
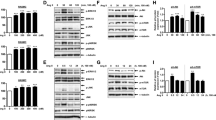
HDO at concentrations of 0.01, 0.1 and 1 μmol·L-1 dose-dependently inhibited VEGF-induced VSMC proliferation with inhibition indices of 6.8 %, 13.1 % and 28.9 %, respectively. Similar concentrations of HDO dose-dependently decreased the percentage of VEGF-induced cells in S phase to 3.6 %, 3.4 %, and 5.4 %, while increasing that of cells arrested in the G0/G1 phase to 80 %, 82 % and 83.6 %. HDO at 0.01, 0.1 or 1 μmol·L-1 inhibited VEGF-induced PKC-α mRNA expression, with inhibition indices of 9.2 %, 16.1 % and 54.0 %. HDO at 0.1 or 1 μmol·L-1 inhibited VEGF-induced proto-oncogene mRNA expression, with inhibition indices of 5.2 % and 6.6 % for c-jun, 8.8 % and 11.6 % for c-myc, and 6.5 % and 11.9 % for c-fos, respectively. Additionally, treatment with 0.01, 0.1 or 1 μmol·L-1 HDO, inhibited VEGF-induced expression of some proliferation related proteins with inhibition indices of 33.2 %, 56.3 % and 77.0 % for PKC-α, 33.7 %, 38.7 % and 53.2 % for p-Akt, 3.5 %, 24.2 % and 49.3 % for p-ERK 1/2, 39.2 %, 71.8 % and 80.7 % for p-PDK 1 and 41.4 %, 89.4 % and 92.4 % for p-GSK-3β, respectively. The results showed that HDO inhibited PKC-α, c-jun, c-fos and c-myc mRNA transcription, and also down-regulated phosphorylation levels of ERK 1/2 and Akt.
Conclusion
Our study demonstrates that HDO inhibits transcription of proliferation-related proto-oncogenes and arrests G1/S transition through inhibition of the PKC, MAPK and Akt/PI3K pathways in association with inhibition of VSMC proliferation. This altered molecular signature may explain one mechanism of HDO-mediated inhibition of VSMC proliferation.






Similar content being viewed by others
References
Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801.
Linares A, Perales S, Palomino-Morales RJ, Castillo M, Alejandre MJ. Nutritional control. gene regulation, and transformation of vascular smooth muscle cells in atherosclerosis. Cardiovasc Hematol Disord: Drug Targets. 2006;6:151–68.
Brown DA, Lee EW, Loh CT, Kee ST. A new wave in treatment of vascular occlusive disease: biodegradable stents-clinical experience and scientific principles. J Vasc Interv Radiol. 2009;20:315–24.
Yun MR, Lee JY, Park HS, et al. Oleic acid enhances vascular smooth muscle cell proliferation via phosphatidylinositol 3-kinase/Akt signaling pathway. Pharmacol Res. 2006;54:97–102.
Lata M, Milhorn DM, Bharati M, Jill S. CD40-mediated activation of vascular smooth muscle cell chemokine production through a Src-initiated, MAPK-dependent pathway. Cell Signal. 2004;16:375–84.
Ettore P, Renata C, Concetta DF, Franco C. Protein kinase C pathway and proliferative responses of aged and young rat vascular smooth muscle cells. Atherosclerosis. 1993;104:137–45.
Beaudry P, Force J, Naumov GN, et al. Differential effects of vascular endothelial growth factor receptor-2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for unease surrogate marker of antiangiogenic activity. Clin Cancer Res. 2005;11:3514–22.
Cucina A, Borrelli V, Randone B, Coluccia P, Sapienza P, Cavallaro A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surgical Res. 2003;109:16–23.
Hoshi S, Nomoto KK, Kuromitsu J, Tomari S, Naqata M. High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem Biophys Res Commun. 2002;290:177–84.
Conti L, Re F, Lazzerini F, et al. Glycosaminoglycan polysulfate (Ateroid) in old-age dementias: effects upon depressive symptomatology in geriatric patients. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:977–81.
Ahmed T, Ungo J, Zhou M, Campo C. Inhibition of allergic late airway responses by inhaled heparin-derived oligosaccharides. J Appl Physiol. 2000;88:1721–9.
Niu J, He SY, Li W, Wang LR. Preparation of heparin derived oligosaccharides and effection of them on vascular smooth muscle cells proliferation. Pharm Biotec. 2009;16:46–9.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein dye-binding. Anal Biochem. 1976;72:248–54.
Sambrook J, Fritsch EF, Maniatis T. Detection and analysis of proteins expressed from cloned genes. In: Molecular Cloning-A Laboratory Manual. Cold Spring Harbor Laboratory Press, 1989. pp. 1847–74.
Kocher O, Skalli O, Cerutti D, Gabbiani F, Gabbiani G. Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res. 1985;56:829–38.
Nilsson J, Sjölund M, Palmberg L, Thyberg J, Heldin CH. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985;82:4418–22.
Little PJ, Ivey ME, Osman N. Endothelin-1 actions on vascular smooth muscle cell functions as a target for the prevention of atherosclerosis. Curr Vasc Pharmacol. 2008;6:195–203.
Min SK, Kenagy RD, Clowes AW. Induction of vascular atrophy as a novel approach to treating restenosis. J Vasc Surg. 2008;47:662–70.
Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–23.
Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–65.
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–92.
Milei J, Grana DR, Navari C, Azzato F, Guerri-Guttenberg RA, Ambrosio G. Coronary intimal thickening in newborn babies and <or=1-year-old infants. Angiology. 2010;61:350–6.
Milei J, Ottaviani G, Lavezzi AM, Grana DR, Stella I, Matturri L. Perinatal and infant early atherosclerotic coronary lesions. Can J Cardiol. 2008;24:137–41.
Pal S, Datta K, Khosravi-Far R, Mukhopadhyay D. Roleof protein kinase Czeta in Ras-mediated transcriptional activation of vascular permeability factor/vascular endothelial growth factor expresstion. J Biol Chem. 2001;276:2395–403.
Jerome-Morais A, Rahn HR, Tibudan SS, Denning MF. Role for protein kinase C-alpha in keratinocyte growth arrest. J Invest Dermatol. 2009;129:2365–75.
Lavezzi AM, Milei J, Grana DR, et al. Expression of c-fos, p53 and PCNA in the unstable atherosclerotic carotid plaque. Int J Cardiol. 2003;92:59–63.
Muller DW. The role of proto-oncogenes in coronary restenosis. Prog Cardiovasc Dis. 1997;40:117–28.
Wang XK, Wang Y, He ZY, Liu GY, Yang CM, Li M. Effects of MAPK antisense oligodeoxy nucleotides on PKC gene expression of human vascular smooth muscle cells induced by insulin. Chin J Appl Physiol. 1999;15:346–9.
The NY, Awards ALM. The family of protein kinase C for signal transduction. JAMA. 1989;262:1826–33.
Suggs WD, Olson SC, Madnani D, et al. Antisense oligonucleotides to c-fos and c-jun inhibit intimal thickening in a rat vein graft model. Surgery. 1999;126:443–9.
Rao GN, Alexander RW, Runge MS. Linoleic acid and its metabolites, hydroperoxyocta decadienoic acids, stimulate c-Fos, c-Jun, and c-Myc mRNA expression, mitogen-activated protein kinase activation, and growth in rat aortic smooth muscle cells. J Clin Invest. 1995;96:842–7.
Mitsumata M, Gamou S, Shimizu N, Yoshida Y. Response of atherosclerotic intimal smooth muscle cells to epidermal growth factor in vitro. Arterioscler Thromb. 1994;14:1364–71.
Schad JF, Meltzer KR, Hicks MR, Beutler DS, Cao TV, Standley PR. Cyclic strain upregulates VEGF and attenuates proliferation of vascular smooth muscle cells. Vasc Cell. 2011;3:21.
Li D, Zhang C, Song F, Lubenec I, Tian Y, Song QH. VEGF regulates FGF-2 and TGF-beta1 expression in injury endothelial cells and mediates smooth muscle cells proliferation and migration. Microvasc Res. 2009;77:134–42.
Acknowledgments
This work was sponsored by the Fundamental Research Funds for the Central Universities (Program Number: JKZ2011013), the Foundation of the Jiangsu Key Laboratory of Molecular and Medical Biotechnology (Grant Number: MMB09KF01), and the Foundation of the Major National Science and Technology Project of China for Significant New Drugs Creation (Grant Number: 2012ZX09502001-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was sponsored by the Fundamental Research Funds for the Central Universities (Program Number: JKZ2011013), the Foundation of the Jiangsu Key Laboratory of Molecular and Medical Biotechnology (Grant Number: MMB09KF01), and the Foundation of the Major National Science and Technology Project of China for Significant New Drugs Creation (Grant Number: 2012ZX09502001-004).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Model of the molecular mechanism of heparin-derived oligosaccharide (HDO) inhibiting vascular endothelial growth factor (vEGF)-induced vascular smooth muscle cell (VSMC) proliferation. (JPEG 30 kb)
Fig. S2
The figure reveals the relationship between HDO and LMWH. Our work of producing HDO has gained the Chinese patent (CN21.201010139398.9). (JPEG 23 kb)
Rights and permissions
About this article
Cite this article
Li, L., Rui, X., Liu, T. et al. Effect of Heparin-Derived Oligosaccharide on Vascular Smooth Muscle Cell Proliferation and the Signal Transduction Mechanisms Involved. Cardiovasc Drugs Ther 26, 479–488 (2012). https://doi.org/10.1007/s10557-012-6419-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-012-6419-8