Abstract
Purpose
Cancer genetic services (counseling/testing) are recommended for women diagnosed with breast cancer younger than 45 years old (young breast cancer survivors—YBCS) and at-risk relatives. We present recruitment of YBCS, identification and recruitment of at-risk relatives, and YBCS willingness to contact their cancer-free, female relatives.
Methods
A random sample of 3,000 YBCS, stratified by race (Black vs. White/Other), was identified through a population-based cancer registry and recruited in a randomized trial designed to increase use of cancer genetic services. Baseline demographic, clinical, and family characteristics, and variables associated with the Theory of Planned Behavior (TPB) were assessed as predictors of YBCS’ willingness to contact at-risk relatives.
Results
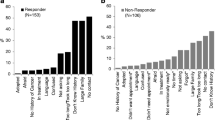
The 883 YBCS (33.2% response rate; 40% Black) who returned a survey had 1,875 at-risk relatives and were willing to contact 1,360 (72.5%). From 853 invited at-risk relatives (up to two relatives per YBCS), 442 responded (51.6% response rate). YBCS with larger families, with a previous diagnosis of depression, and motivated to comply with recommendations from family members were likely to contact a greater number of relatives. Black YBCS were more likely to contact younger relatives and those living further than 50 miles compared to White/Other YBCS.
Conclusion
It is feasible to recruit diverse families at risk for hereditary cancer from a population-based cancer registry. This recruitment approach can be used as a paradigm for harmonizing processes and increasing internal and external validity of large-scale public health genomic initiatives in the era of precision medicine.


Similar content being viewed by others
References
Bowen MS, Kolor K, Dotson WD, Ned RM, Khoury MJ (2012) Public health action in genomics is now needed beyond newborn screening. Public Health Genom 15(6):327–334. doi:10.1159/000341889
Balmana J, Diez O, Rubio IT, Cardoso F (2011) BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol 22(Suppl 6):vi31–34. doi:10.1093/annonc/mdr373
Couch FJ, Nathanson KL, Offit K (2014) Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343(6178):1466–1470. doi:10.1126/science.1251827
Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R (2014) Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med 160(4):255–266. doi:10.7326/M13-1684
NCCN (2016) Genetic/familal high-risk assessment: breast and ovarian. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 25 Nov 2016
Bastani R, Maxwell AE, Bradford C, Das IP, Yan KX (1999) Tailored risk notification for women with a family history of breast cancer. Prev Med 29(5):355–364. doi:10.1006/pmed.1999.0556
Pal T, Rocchio E, Garcia A, Rivers D, Vadaparampil S (2011) Recruitment of black women for a study of inherited breast cancer using a cancer registry-based approach. Genet Test Mol Biomarkers 15(1–2):69–77. doi:10.1089/gtmb.2010.0098
Simmons RG, Lee YC, Stroup AM, Edwards SL, Rogers A, Johnson C, Wiggins CL, Hill DA, Cress RD, Lowery J, Walters ST, Jasperson K, Higginbotham JC, Williams MS, Burt RW, Schwartz MD, Kinney AY (2013) Examining the challenges of family recruitment to behavioral intervention trials: factors associated with participation and enrollment in a multi-state colonoscopy intervention trial. Trials 14:116. doi:10.1186/1745-6215-14-116
Smith T, Stein KD, Mehta CC, Kaw C, Kepner JL, Buskirk T, Stafford J, Baker F (2007) The rationale, design, and implementation of the American Cancer Society’s studies of cancer survivors. Cancer 109(1):1–12. doi:10.1002/cncr.22387
Courtney RJ, Paul CL, Carey ML, Sanson-Fisher RW, Macrae FA, D’Este C, Hill D, Barker D, Simmons J (2013) A population-based cross-sectional study of colorectal cancer screening practices of first- degree relatives of colorectal cancer patients. BMC Cancer 13(1):13
Dite GS, Jenkins MA, Southey MC, Hocking JS, Giles GG, McCredie MR, Venter DJ, Hopper JL (2003) Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst 95(6):448–457
Lijovic M, Davis SR, Fradkin P, La China M, Farrugia H, Wolfe R, Bell RJ (2008) Use of a cancer registry is preferable to a direct-to-community approach for recruitment to a cohort study of wellbeing in women newly diagnosed with invasive breast cancer. BMC Cancer 8:126. doi:10.1186/1471-2407-8-126
Sutherland HJ, Lacroix J, Knight J, Andrulis IL, Boyd NF, Ontario Cancer Genetics, N (2001) The Cooperative Familial Registry for Breast Cancer Studies: design and first year recruitment rates in Ontario. J Clin Epidemiol 54(1):93–98
Carpentier MY, Tiro JA, Savas LS, Bartholomew LK, Melhado TV, Coan SP, Argenbright KE, Vernon SW (2013) Are cancer registries a viable tool for cancer survivor outreach? A feasibility study. J Cancer Surviv 7(1):155–163. doi:10.1007/s11764-012-0259-1
Pakilit AT, Kahn BA, Petersen L, Abraham LS, Greendale GA, Ganz PA (2001) Making effective use of tumor registries for cancer survivorship research. Cancer 92(5):1305–1314
Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, Limburg P, Lindor N, Potter JD, Templeton AS, Thibodeau S, Seminara D, Colon Cancer Family R (2007) Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev 16(11):2331–2343. doi:10.1158/1055-9965.EPI-07-0648
Carey M, Sanson-Fisher R, Macrae F, Hill D, D’Este C, Paul C, Doran C (2012) Improving adherence to surveillance and screening recommendations for people with colorectal cancer and their first degree relatives: a randomized controlled trial. BMC Cancer 12. doi:10.1186/1471-2407-12-62, Artn 62
Anderson B, McLosky J, Wasilevich E, Lyon-Callo S, Duquette D, Copeland G (2012) Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol 2012:298745. doi:10.1155/2012/298745
Knight JA, Sutherland HJ, Glendon G, Boyd NF, Andrulis IL, Network OCG (2002) Characteristics associated with participation at various stages at the Ontario site of the Cooperative Family Registry for Breast Cancer Studies. Ann Epidemiol 12(1):27–33. doi:Doi:10.1016/S1047-2797(01)00253-8
Katapodi MC, Northouse LL, Schafenacker AM, Duquette D, Duffy SA, Ronis DL, Anderson B, Janz NK, McLosky J, Milliron KJ, Merajver SD, Duong LM, Copeland G (2013) Using a state cancer registry to recruit young breast cancer survivors and high-risk relatives: protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer. 13. doi:10.1186/1471-2407-13-97, Artn 97
Wikipedia (2012) Stratified Sampling. WIKI. https://en.wikipedia.org/wiki/Stratified_sampling.
Beskow LM, Sandler RS, Millikan RC, Weinberger M (2005) Patient perspectives on research recruitment through cancer registries. Cancer Causes Control 16(10):1171–1175. doi:10.1007/s10552-005-0407-2
Beskow LM, Millikan RC, Sandler RS, Godley PA, Weiner BJ, Weinberger M (2006) The effect of physician permission versus notification on research recruitment through cancer registries (United States). Cancer Causes Control 17(3):315–323. doi:10.1007/s10552-005-0521-1
Ajzen I (1991) The Theory of Planned Behavior. Organ Behav Hum Decis Process 50(2):179–211. doi:10.1016/0749-5978(91)90020-T
Katapodi MC, Northouse LL, Milliron KJ, Liu G, Merajver SD (2013) Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psychooncology 22(6):1336–1343. doi:10.1002/pon.3139
Su Y-S, Yajima M, Gelman AE, Hill J (2011) Multiple imputation with diagnostics (mi) in R: Opening windows into the black box. J Stat Softw 45(2):1–31
Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33(1):1–22
Tibshirani R (1997) The lasso method for variable selection in the Cox model. Stat Med 16(4):385–395
Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley series in probability and mathematical statistics applied probability and statistics. Wiley, New York
Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN (2014) Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw 12(9):1339–1346
Hamann HA, Tiro JA, Sanders JM, Melhado TV, Funk RK, Carpentier MY, Bartholomew LK, Argenbright KE, Vernon SW (2013) Validity of self-reported genetic counseling and genetic testing use among breast cancer survivors. J Cancer Surviv 7(4):624–629. doi:10.1007/s11764-013-0301-y
Collins FS, Varmus H (2015) A new initiative on precision medicine. N Engl J Med 372(9):793–795
Khoury MJ, Bowen S, Bradley LA, Coates R, Dowling NF, Gwinn M, Kolor K, Moore CA, St Pierre J, Valdez R, Yoon PW (2009) A decade of public health genomics in the United States: centers for disease control and prevention 1997–2007. Public Health Genom 12(1):20–29. doi:10.1159/000153427
Syurina EV, Brankovic I, Probst-Hensch N, Brand A (2011) Genome-based health literacy: a new challenge for public health genomics. Public Health Genom 14(4–5):201–210. doi:10.1159/000324238
Boccia S, Mc Kee M, Adany R, Boffetta P, Burton H, Cambon-Thomsen A, Cornel MC, Gray M, Jani A, Knoppers BM, Khoury MJ, Meslin EM, Van Duijn CM, Villari P, Zimmern R, Cesario A, Puggina A, Colotto M, Ricciardi W (2014) Beyond public health genomics: proposals from an international working group. Eur J Public Health 24(6):877–879. doi:10.1093/eurpub/cku142
Modell SM, Greendale K, Citrin T, Kardia SL Expert and advocacy group consensus findings on the horizon of public health genetic testing. In: Healthcare, 2016. vol 1. Multidisciplinary Digital Publishing Institute, p 14
Acknowledgments
Jenna McLoskly, CGC, Cancer-Genomics Program—Michigan Department of Health and Human Services for patient and at-risk relative identification, recruitment, and assessment of eligibility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was supported by the Centers for Disease Control and Prevention (CDC), 5U48DP001901-03 and by the Robert Wood Johnson Foundation (RWJF)—Nurse Faculty Scholars 68039 Award.
Conflict of interest
Maria C. Katapodi was the Principal Investigator of this study and received research support from DCD and RWJF. Debra Duquette declares that she has no conflict of interest James J. Yang declares that he has no conflict of interest. Kari Mendelsohn-Victor declares that she has no conflict of interest Beth Anderson declares that she has no conflict of interest. Christos Nikolaidis declares that he has no conflict of interest Emily Mancewicz declares that she has no conflict of interest Laurel L. Northouse declares that she has no conflict of interest Sonia Duffy declares that she has no conflict of interest. David Ronis declares that he has no conflict of interest Kara J. Milliron declares that she has no conflict of interest. Nicole Probst-Herbst declares that she has no conflict of interest Sofia D. Merajver declares that she has no conflict of interest. Nancy K. Janz declares that she has no conflict of interest. Glenn Copeland declares that he has no conflict of interest. J. Scott Roberts declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Katapodi, M.C., Duquette, D., Yang, J.J. et al. Recruiting families at risk for hereditary breast and ovarian cancer from a statewide cancer registry: a methodological study. Cancer Causes Control 28, 191–201 (2017). https://doi.org/10.1007/s10552-017-0858-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-017-0858-2