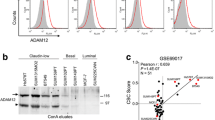
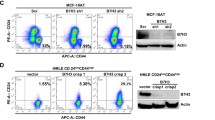
Abstract
Purpose
The CD44+/CD24− cell phenotype is enriched in triple negative breast cancers, is associated with tumor invasive properties, and serves as a cell surface marker profile of breast cancer stem-like cells. Activation of Epidermal Growth Factor Receptor (EGFR) promotes the CD44+/CD24− phenotype, but the specific signaling pathway downstream of EGFR responsible for this effect is not clear. The purpose of this study was to determine the role of the MEK/ERK pathway in the expansion of CD44+/CD24− populations in TNBC cells in response to EGFR activation.
Methods
Representative TNBC cell lines SUM159PT (claudin-low) and SUM149PT (basal) were used to evaluate cell surface expression of CD44 and CD24 by flow cytometry in response to EGFR and MEK inhibition or activation. EGFR and ERK phosphorylation levels were analyzed by Western blotting. The relationship between EGFR phosphorylation and MEK activation score in basal and claudin-low tumors from the TCGA database was examined.
Results
Inhibition of ERK activation with selumetinib, a MEK1/2 inhibitor, blocked EGF-induced expansion of CD44+/CD24− populations. Sustained activation of ERK by overexpression of constitutively active MEK1 was sufficient to expand CD44+/CD24− populations in cells in which EGFR activity was blocked by either erlotinib, an EGFR kinase inhibitor, or BB-94, a metalloprotease inhibitor that prevents generation of soluble EGFR ligands. In basal and claudin-low tumors from the TCGA database, there was a positive correlation between EGFR_pY1068 and MEK activation score in tumors without genomic loss of DUSP4, a negative regulator of ERK, but not in tumors harboring DUSP4 deletion.
Conclusion
Our results demonstrate that ERK activation is a key event in EGFR-dependent regulation of CD44+/CD24− populations. Furthermore, our findings highlight the role of ligand-mediated EGFR signaling in the control of MEK/ERK pathway output in TNBC tumors without DUSP4 loss.








Similar content being viewed by others
Abbreviations
- TNBC:
-
Triple negative breast cancer
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- HER2:
-
Human epidermal growth factor receptor 2
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- MEK:
-
Mitogen-activated protein kinase kinase
- MAPK:
-
Mitogen-activated protein kinase
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
Jun N-terminal kinase
- DUSP4:
-
Dual specificity phosphatase 4
- EMT:
-
Epithelial-to-mesenchymal transition
- CSCs:
-
Cancer stem cells
- FACS:
-
Fluorescence-activated cell sorting
- PE:
-
Phycoerythrin
- APC:
-
Allophycocyanin
- GEO:
-
Gene expression omnibus
- TCGA:
-
The Cancer Genome Atlas
- GISTIC:
-
Genomic identification of significant targets in cancer
References
Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT (2012) Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat 136:331–345
Williams CB, Soloff AC, Ethier SP, Yeh ES (2015) Perspectives on epidermal growth factor receptor regulation in triple-negative breast cancer: ligand-mediated mechanisms of receptor regulation and potential for clinical targeting. Adv Cancer Res 127:253–281
Hsu JL, Hung MC (2016) The role of HER2, EGFR, and other receptor tyrosine kinases in breast cancer. Cancer Metastasis Rev 35:575–588
Verbeek BS, Adriaansen-Slot SS, Vroom TM, Beckers T, Rijksen G (1998) Overexpression of EGFR and c-erbB2 causes enhanced cell migration in human breast cancer cells and NIH3T3 fibroblasts. FEBS Lett 425:145–150
Mukhopadhyay P, Lakshmanan I, Ponnusamy MP, Chakraborty S, Jain M, Pai P et al (2013) MUC4 overexpression augments cell migration and metastasis through EGFR family proteins in triple negative breast cancer cells. PLoS ONE 8:e54455
Maretzky T, Evers A, Zhou W, Swendeman SL, Wong PM, Rafii S et al (2011) Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun 2:229
Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J et al (2003) Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol 5:733–740
Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N (2006) Molecular pathways regulating EGF-induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol 290:C1532–C1542
Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y et al (2007) Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67:9066–9076
Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P et al (2009) Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res 15:6639–6648
Bartholomeusz C, Xie X, Pitner MK, Kondo K, Dadbin A, Lee J et al (2015) MEK inhibitor Selumetinib (AZD6244; ARRY-142886) prevents lung metastasis in a triple-negative breast cancer xenograft model. Mol Cancer Ther 14:2773–2781
Wang M, Kern AM, Hulskotter M, Greninger P, Singh A, Pan Y et al (2014) EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation. Cancer Res 74:2825–2834
Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G et al (2013) EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell 154:1269–1284
Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K et al (2008) The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 10:R53
Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM (2013) Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 18:123–133
Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA et al (2010) Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44hi/CD24lo/− stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 15:235–252
Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH et al (2006) CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res 8:R59
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988
Tanei T, Choi DS, Rodriguez AA, Liang DH, Dobrolecki L, Ghosh M et al (2016) Antitumor activity of Cetuximab in combination with Ixabepilone on triple negative breast cancer stem cells. Breast Cancer Res 18:6
Henson E, Chen Y, Gibson S (2017) EGFR family members’ regulation of autophagy is at a crossroads of cell survival and death in cancer. Cancers (Basel) 9:27
Chen Y, Henson ES, Xiao W, Huang D, McMillan-Ward EM, Israels SJ et al (2016) Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 12:1029–1046
Fung C, Chen X, Grandis JR, Duvvuri U (2012) EGFR tyrosine kinase inhibition induces autophagy in cancer cells. Cancer Biol Ther 13:1417–1424
Li X, Fan Z (2010) The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1α and Bcl-2 and activating the beclin 1/hVps34 complex. Cancer Res 70:5942–5952
Balko JM, Schwarz LJ, Bhola NE, Kurupi R, Owens P, Miller TW et al (2013) Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res 73:6346–6358
Morrison DK (2012) MAP kinase pathways. Cold Spring Harb Perspect Biol 4:a011254
Blobel CP (2005) ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6:32–43
Kataoka H (2009) EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. J Dermatol Sci 56:148–153
Giltnane JM, Balko JM (2014) Rationale for targeting the Ras/MAPK pathway in triple-negative breast cancer. Discov Med 17:275–283
Mazumdar A, Poage GM, Shepherd J, Tsimelzon A, Hartman ZC, Den Hollander P et al (2016) Analysis of phosphatases in ER-negative breast cancers identifies DUSP4 as a critical regulator of growth and invasion. Breast Cancer Res Treat 158:441–454
Creighton CJ, Hilger AM, Murthy S, Rae JM, Chinnaiyan AM, El-Ashry D (2006) Activation of mitogen-activated protein kinase in estrogen receptor & #x03B1;-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor & #x03B1;-negative human breast tumors. Cancer Res 66:3903–3911
Bhat-Nakshatri P, Appaiah H, Ballas C, Pick-Franke P, Goulet R Jr, Badve S et al (2010) SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24− phenotype. BMC Cancer 10:411
The Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490:61–70
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–404
Li H, Duhachek-Muggy S, Qi Y, Hong Y, Behbod F, Zolkiewska A (2012) An essential role of metalloprotease-disintegrin ADAM12 in triple-negative breast cancer. Breast Cancer Res Treat 135:759–769
Li H, Duhachek-Muggy S, Dubnicka S, Zolkiewska A (2013) Metalloproteinase-disintegrin ADAM12 is associated with a breast tumor-initiating cell phenotype. Breast Cancer Res Treat 139:691–703
Duhachek-Muggy S, Qi Y, Wise R, Alyahya L, Li H, Hodge J et al (2017) Metalloprotease-disintegrin ADAM12 actively promotes the stem cell-like phenotype in claudin-low breast cancer. Mol Cancer 16:32
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A et al (2009) Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 106:13820–13825
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI et al (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12:R68
Haglund K, Dikic I (2012) The role of ubiquitylation in receptor endocytosis and endosomal sorting. J Cell Sci 125:265–275
Sorkin A, Goh LK (2009) Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res 315:396–683
Hollestelle A, Elstrodt F, Nagel JH, Kallemeijn WW, Schutte M (2007) Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol Cancer Res 5:195–201
Fillmore CM, Kuperwasser C (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 10:R25
Keller PJ, Arendt LM, Skibinski A, Logvinenko T, Klebba I, Dong S et al (2011) Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci USA 109:2772–2777
Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF et al (2007) Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129:1065–1079
Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A et al (2014) Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509:492–496
Nyati MK, Morgan MA, Feng FY, Lawrence TS (2006) Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer 6:876–885
Shostak K, Chariot A (2015) EGFR and NF-κB: partners in cancer. Trends Mol Med 21:385–393
Baselga J, Gomez P, Greil R, Braga S, Climent MA, Wardley AM et al (2013) Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol 31:2586–2592
Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX et al (2012) TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol 30:2615–2623
Tredan O, Campone M, Jassem J, Vyzula R, Coudert B, Pacilio C et al (2015) Ixabepilone alone or with cetuximab as first-line treatment for advanced/metastatic triple-negative breast cancer. Clin Breast Cancer 15:8–15
Ueno NT, Zhang D (2011) Targeting EGFR in triple negative breast cancer. J Cancer 2:324–328
Costa R, Shah AN, Santa-Maria CA, Cruz MR, Mahalingam D, Carneiro BA et al (2017) Targeting Epidermal Growth Factor Receptor in triple negative breast cancer: new discoveries and practical insights for drug development. Cancer Treat Rev 53:111–119
Nakai K, Hung MC, Yamaguchi H (2016) A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res 6:1609–1623
Fleisher B, Clarke C, Ait-Oudhia S (2016) Current advances in biomarkers for targeted therapy in triple-negative breast cancer. Breast Cancer 8:183–197
Farnie G, Clarke RB, Spence K, Pinnock N, Brennan K, Anderson NG et al (2007) Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst 99:616–627
Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Vellon L, Menendez JA (2011) Autophagy positively regulates the CD44(+) CD24(-/low) breast cancer stem-like phenotype. Cell Cycle 10:3871–3885
Choi DS, Blanco E, Kim YS, Rodriguez AA, Zhao H, Huang TH et al (2014) Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells 32:2309–2323
Liang DH, Choi DS, Ensor JE, Kaipparettu BA, Bass BL, Chang JC (2016) The autophagy inhibitor chloroquine targets cancer stem cells in triple negative breast cancer by inducing mitochondrial damage and impairing DNA break repair. Cancer Lett 376:249–258
Qiang L, Zhao B, Ming M, Wang N, He TC, Hwang S et al (2014) Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proc Natl Acad Sci USA 111:9241–9246
Qiang L, He YY (2014) Autophagy deficiency stabilizes TWIST1 to promote epithelial-mesenchymal transition. Autophagy 10:1864–1865
Bayliss J, Hilger A, Vishnu P, Diehl K, El-Ashry D (2007) Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res 13:7029–7036
Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P et al (2016) RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 22:1499–1509
Acknowledgements
This work was supported by NIH Grant R01CA172222 to AZ. This is contribution 17-394-J from Kansas Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wise, R., Zolkiewska, A. Metalloprotease-dependent activation of EGFR modulates CD44+/CD24− populations in triple negative breast cancer cells through the MEK/ERK pathway. Breast Cancer Res Treat 166, 421–433 (2017). https://doi.org/10.1007/s10549-017-4440-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4440-0