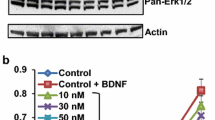
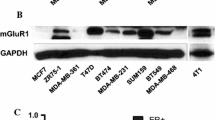
Abstract
We investigated the role of glial cell line-derived neurotrophic factor (GDNF) in compensating trastuzumab (TZMB)-induced apoptosis in HER2+ breast cancer (BC) cells using xenograft tumors. We generated BC xenografts in nude mice using samples from three patients selected based on their HER2 status and response to TZMB therapy. TZMB treatment resulted in shrinkage of the HER2+ TZMB-sensitive xenograft tumor but not the HER2− or HER2+ TZMB-resistant ones. GDNF neutralized TZMB activity and induced growth in all tumors. Three distinct cell lines were derived from these tumors and named, respectively, TZMB-sensitive (TSTC), HER2− (HNTC), and TZMB-resistant (TRTC). Over 50% of TRTC but 1% of TSTC cells expressed CD44, whereas 84% of TSTC were CD24+ compared to only 1% of TRTC, despite comparable levels of HER2 detected in both. TZMB induced profound morphological changes toward apoptosis in TSTC but not in TRTC or HNTC. However, GDNF significantly compensated TZMB-mediated TSTC cell loss and promoted growth by 37 and 50%, respectively, in TSTC and TRTC. Inhibition of SRC by Saracatinib (SARC) blocked GDNF function and accelerated TZMB-mediated cell death in TSTC, but GDNF continued promoting TRTC growth. These changes paralleled with expression levels of the key molecules involved in growth and apoptosis. Collectively, we found in our xenograft samples that firstly SRC mediates GDNF pro-survival functions by bridging RET–HER2 crosstalk in TZMB-responsive BC tumors. Secondly, SARC–TZMB interactions can synergistically eradicate such tumor cells; and thirdly, GDNF can support antibody resistance by acting independent from SRC in tumors with poor HER2 response to TZMB therapy.





Similar content being viewed by others
References
Takeuchi K, Ito F (2011) Receptor tyrosine kinases and targeted cancer therapeutics. Biol Pharm Bull 34(12):1774–1780
Yarden Y (2001) The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer 37(Suppl 4):S3–S8
Chang JC (2007) HER2 inhibition: from discovery to clinical practice. Clin Cancer Res 13:1–3
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Oliveras-Ferraros C, Vazquez-Martin A, Martin-Castilló B, Pérez-Martínez MC, Cufí S, Del Barco S et al (2010) Pathway-focused proteomic signatures in HER2-overexpressing breast cancer with a basal-like phenotype: new insights into de novo resistance to trastuzumab (Herceptin). Int J Oncol 37(3):669–678
Nahta R, Esteva FJ (2006) Herceptin: mechanisms of action and resistance. Cancer Lett 232(2):123–138
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11):783–792
Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23(19):4265–4274
Pohlmann PR, Mayer IA, Mernaugh R (2009) Resistance to trastuzumab in breast cancer. Clin Cancer Res 15:7479–7491
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M et al (2003) The HER-2/neu gene and protein in breast cancer: biomarker and target of therapy. Oncol 8:307–325
Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH (2002) Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer 97:306–312
Osborne CK, Shou J, Massarweh S, Schiff R (2005) Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 11(2 Pt 2):865s–870s
Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935
Boulay A, Breuleux M, Stephan C, Fux C, Brisken C, Fiche M et al (2008) The Ret receptor tyrosine kinase pathway functionally interacts with the ERalpha pathway in breast cancer. Cancer Res 68:3743–3751
Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M et al (2008) Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456(7222):663–666
Morandi A, Martin L-A, Gao Q, Pancholi S, Mackay A, Robertson D et al (2013) GDNF–RET signaling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Res 73:3783–3795
Plaza-Menacho I, Morandi A, Robertson D, Pancholi S, Drury S, Dowsett M et al (2010) Targeting the receptor tyrosine kinase RET sensitizes breast cancer cells to tamoxifen treatment and reveals a role for RET in endocrine resistance. Oncogene 29:4648–4657
Esseghir S, Todd SK, Hunt T, Poulsom R, Plaza-Menacho I, Reis-Filho JS et al (2007) A role for glial cell derived neurotrophic factor induced expression by inflammatory cytokines and RET/GFR alpha 1 receptor up-regulation in breast cancer. Cancer Res 67(24):11732–11741
Sariola H, Saarma M (2003) Novel functions and signalling pathways for GDNF. J Cell Sci 116:3855–3862
Drosten M, Putzer BM (2006) Mechanisms of disease: cancer targeting and the impact of oncogenic RET for medulary thyroid carcinoma therapy. Nat Clin Pract Oncol 3:564–574
Parsons SJ, Parsons JT (2004) Src family kinases, key regulators of signal transduction. Oncogene 23:7906–7909
Biscardi JS, Belsches AP, Parsons SJ (1998) Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog 21(4):261–272
Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, Rijksen G (1996) c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis. J Pathol 180(4):383–388
Myoui A, Nishimura R, Williams PJ, Hiraga T, Tamura D, Michigami T et al (2003) C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res 63:5028–5033
Schmitz KJ, Grabellus F, Callies R, Otterbach F, Wohlschlaeger J, Levkau B et al (2005) High expression of focal adhesion kinase (p125FAK) in node-negative breast cancer is related to overexpression of HER-2/neu and activated Akt kinase but does not predict outcome. Breast Cancer Res 7(2):R194–R203
Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW et al (2011) Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 17(4):461–469
Peiró G, Ortiz-Martínez F, Gallardo A, Pérez-Balaguer A, Sánchez-Payá J, Ponce JJ et al (2014) Src, a potential target for overcoming trastuzumab resistance in HER2-positive breast carcinoma. Br J Cancer 111(4):689–695
Gardaneh M, Shojaei S (2013) The anti-apoptotic impact of neurotrophic factor GDNF on breast cancer cells pre-treated with trastuzumab. Proceedings: AACR 104th Annual Meeting, Washington, DC (Abstract 969)
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412
Gardaneh M, Gholami M, Maghsoudi N (2011) Synergy between glutathione peroxidase-1 and astrocytic growth factors suppresses free radical generation and protects dopaminergic neurons against 6-hydroxydopamine. Rejuv Res 14:195–204
Safi R, Gardaneh M, Panahi Y, Maghsoudi N, Zaefizadeh M, Gharib E (2012) Optimized quantities of GDNF overexpressed by engineered astrocytes are critical for protection of neuroblastoma cells against 6-OHDA toxicity. J Mol Neurosci 46:654–665
Gharib E, Gardaneh M, Shojaei S (2013) Upregulation of glutathione peroxidase-1 expression and activity by glial cell line-derived neurotrophic factor promotes high-level protection of PC12 cells against 6-hydroxydopamine and hydrogen peroxide toxicities. Rejuv Res 16:185–199
Esmaeilzadeh E, Gardaneh M, Gharib E, Sabouni F (2013) Shikonin protects dopaminergic cell line PC12 against 6-hydroxydopamine-mediated neurotoxicity via both glutathione-dependent and independent pathways and by inhibiting apoptosis. Neurochem Res 38:1590–1604
Richmond A, Su Y (2008) Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech 1(2–3):78–82
Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M et al (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139(2):539–552
Boulbes DR, Chauhan GB, Jin Q, Bartholomeuz C, Esteva FJ (2015) CD44 expression contributes to trastuzumab resistance in HER2-positive breast cancer cells. Breast Cancer Res Treat 151(3):501–513
Summy JM, Gallick GE (2003) Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev 22:337–358
Acknowledgement
We thank those patients who contributed to this study by donating their tumor samples. We also appreciate collaborations made by staff in Imam Khomeini Hospital in Tehran for generation of xenograft models of tumors. This work was supported by a Grant (II-502) from NIGEB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of our Institutional Ethics Committee, Ministry of Health in Iran and the 1964 Helsinki declaration and its later amendments. Also, all applicable international, national guidelines, the ARRIVE guidelines [29] besides those set by our Institutional Ethical Committee for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gardaneh, M., Shojaei, S., Kaviani, A. et al. GDNF induces RET–SRC–HER2-dependent growth in trastuzumab-sensitive but SRC-independent growth in resistant breast tumor cells. Breast Cancer Res Treat 162, 231–241 (2017). https://doi.org/10.1007/s10549-016-4078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4078-3