Abstract
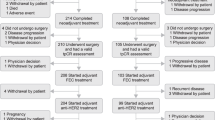
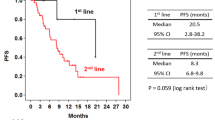
We previously reported progression-free survival (PFS) results on a phase II trial of weekly paclitaxel, trastuzumab, and pertuzumab in patients with human epidermal growth factor receptor 2(HER2)–positive metastatic breast cancer (MBC) treated in the first- and second-line setting. Here, we report results for overall survival (OS) and updated PFS after an additional year of follow-up. Patients with HER2-positive MBC with 0–1 prior treatment were eligible. Treatment consisted of paclitaxel (80 mg/m2) weekly, and trastuzumab (loading dose 8 mg/kg → 6 mg/kg) and pertuzumab (loading dose 840 mg → 420 mg) every 3 weeks, all given intravenously. Primary endpoint was 6-month PFS. Secondary endpoints included median PFS, 6-month and median OS. Evaluable patients received at least one full dose of treatment. From January 2011 to December 2013, 69 patients were enrolled: 51 (74 %) and 18 (26 %) treated in first- and second-line metastatic settings, respectively. As of July 1, 2015, the median follow-up was 33 months (range 3–49 months; 67 patients were evaluable for efficacy). The median OS was 44 months (95 % CI 37.5–NR) overall and 44 months (95 % CI 38.3–NR) and 37.5 months (95 % CI 30.3–NR) for patients with 0 and 1 prior metastatic treatment, respectively; 6-month OS was 98 % (95 % CI 90-1). The 6-month PFS was 86 % (95 % CI 75–93) overall and 89 % (95 % CI 76–95) and 78 % (95 % CI 51–91) for patients with 0 and 1 prior therapy, respectively; and median PFS was 21.4 months (95 % CI 14.1–NR) overall and 25.7 months (95 % CI 14.1–NR) and 16.9 months (95 % CI 8.5–NR) for patients with 0–1 prior treatment, respectively. Treatment was well tolerated. Updated analysis demonstrates that weekly paclitaxel, when added to trastuzumab and pertuzumab, is associated with a favorable OS and PFS and offers an alternative to docetaxel-based therapy. http://www.ClinicalTrials.gov NCT0127604




Similar content being viewed by others
References
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY) 235(4785):177–182
Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L et al (1998) neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16(4):1340–1349
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M et al (2001) Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N Engl J Med 344(11):783–792
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A et al (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119
Hudis CA (2007) Trastuzumab— Mechanism of Action and Use in Clinical Practice. N Engl J Med 357(1):39–51
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX (2004) Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5(4):317–328
Baselga J, Swain SM (2009) Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 9(7):463–475
Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K et al (2002) Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2(2):127–137
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M (2009) Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 69(24):9330–9336
Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A et al (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14(6):461–471
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358(16):1663–1671
Dang C, Iyengar N, Datko F, D’Andrea G, Theodoulou M, Dickler M, Goldfarb S, Lake D, Fasano J, Fornier M et al (2015) Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 33(5):442–447
Gradishar WJA, Benjamin O (2015) Balassanian, Ron Blair et al.: Breast Cancer Version 2.2015. J Natl Compr Canc Netw 13(4):448–475
Yu AF, Manrique C, Pun S, Liu JE, Mara E, Fleisher M, Patil S, Jones LW, Steingart RM, Hudis CA et al (2016) Cardiac Safety of Paclitaxel Plus Trastuzumab and Pertuzumab in Patients With HER2-Positive Metastatic Breast Cancer. The Oncologist 21(4):418–424
Andersson M, López-Vega JM, Petit T, Zamagni C, Donica M, Kamber J, Perez EA (2015) The co-administration of pertuzumab (P) and trastuzumab (T) as a single infusion, followed by vinorelbine (V), in first-line (1L) treatment of HER2-positive locally advanced or metastatic breast cancer (MBC) patients (pts): VELVET study interim analysis. J Clin Oncol 33(15):586 2015 ASCO Annual Meeting
Perez EA, Lopez-Vega JM, Mastro LD, Petit T, Mitchell L, Pelizon CH, Andersson M (2012) A combination of pertuzumab, trastuzumab, and vinorelbine for first-line treatment of patients with HER2-positive metastatic breast cancer: An open-label, two-cohort, phase II study (VELVET). J Clin Oncol 30(15):TPS653 2012 ASCO Annual Meeting
Andersson M, Lopez-Vega JM, Petit T, Zamagni C, Freudensprung U, Robb S, Restuccia E, Perez EA (2014) 361PDINTERIM SAFETY AND EFFICACY OF PERTUZUMAB, TRASTUZUMAB AND VINORELBINE FOR FIRST-LINE (1L) TREATMENT OF PATIENTS (PTS) WITH HER2-POSITIVE LOCALLY ADVANCED OR METASTATIC BREAST CANCER (MBC). Annals of Oncology 25(4):iv120–iv120
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32
Amiri-Kordestani L, Wedam S, Zhang L, Tang S, Tilley A, Ibrahim A, Justice R, Pazdur R, Cortazar P (2014) First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 20(21):5359–5364
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 24(9):2278–2284
Von Minckwitz G, Baselga J, Bradbury I, de Azambuja E, Scullion M, Ross G, Saini K, Piccart-Gebhart M (2011) Adjuvant Pertuzumab and Herceptin IN IniTial TherapY of Breast Cancer: APHINITY (BIG 4–11/BO25126/TOC4939 g). Cancer Research 71(24 Supplement):OT1-02–04
A Study of Kadcyla (TrastuzumabEmtansine) Plus Perjeta (Pertuzumab) Following Anthracyclines in Comparison With Herceptin (Trastuzumab) Plus Perjeta and a Taxane Following Anthracyclines as Adjuvant Therapy in Patients With Operable HER2-Positive Primary Breast Cancer. http://clinicaltrials.gov/ct2/show/NCT01966471
Ellis PA, Barrios CH, Eiermann W, Toi M, Im Y-H, Conte PF, Martin M, Pienkowski T, Pivot XB, Burris HA et al (2015) Phase III, randomized study of trastuzumab emtansine (T-DM1) ± pertuzumab (P) vs trastuzumab + taxane (HT) for first-line treatment of HER2-positive MBC: Primary results from the MARIANNE study. J Clin Oncol 33(15):507
Acknowledgments
We thank all the study investigators and their clinical teams for their contribution to this study, and the patients for agreeing to participate.
Funding
This work was supported by Roche-Genentech. No grant applied.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosures
LMS, NMI, MFC, SMP, SP, CWL, DFA, JCS, SC, SMS, EAC, PD, TTS, JB, LN: no COI.CAH: Consulting- Genentech, CTD: Research funding-Genentech/Roche. TAT: Advisory–Genentech.DFA: Travel- Roche.
Rights and permissions
About this article
Cite this article
Smyth, L.M., Iyengar, N.M., Chen, M.F. et al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2-overexpressing metastatic breast cancer: overall survival and updated progression-free survival results from a phase II study. Breast Cancer Res Treat 158, 91–97 (2016). https://doi.org/10.1007/s10549-016-3851-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3851-7