Abstract
In spite of its demonstrated benefits, many women do not initiate hormonal therapy, and among those who do, many discontinue it prematurely. We examined whether differences in hormonal therapy adherence may be at least partially explained by the availability of prescription drug coverage. Women aged 20–79 years diagnosed with stage I-III breast cancer between June 2005 and February 2007 were enrolled in the study. Women completed a mailed survey, on average 9 months after diagnosis, and again approximately 4 years later (N = 712). Adjusted logistic regression was used to predict the likelihood of initiating hormonal therapy and hormonal therapy continuation. Women who had prescription drug coverage were more likely to initiate hormonal therapy relative to women without prescription drug coverage (OR 2.91, 95 % CI 1.24–6.84). Women with prescription drug coverage were also more likely to continue hormonal therapy (OR 2.23; 95 % CI 0.99–5.05, p = 0.0543). The lowest income women were also less likely to continue hormonal therapy relative to women with annual household income that exceeded $70,000 (OR 0.55; 95 % CI 0.29–1.04) with a borderline significance of (p = 0.08). This study demonstrates the critical role of prescription drug coverage in hormonal therapy initiation and continuation, independent of health insurance coverage. These findings add to the body of literature that addresses medication adherence. Financial factors must be considered along with behavioral factors that influence adherence, which is becoming increasingly relevant to oncology as treatments are shifted to oral medications, many of which are very expensive.
Similar content being viewed by others
Introduction
Hormonal (endocrine) therapy is an important component of hormone-receptor positive breast cancer treatment. Adherence to hormonal therapy recommendations is shown to prevent breast cancer recurrence and improve survival [1–4]. These medications can result in a 50 % reduction in breast cancer recurrence in women at high risk for recurrence [5]. However, in spite of its demonstrated benefits, many women do not initiate hormonal therapy, and among those who do, many discontinue it before the full 5–10 years recommended following surgery for breast cancer [6–9].
Women who fail to initiate hormonal therapy or discontinue it prematurely are at considerably greater risk for recurrence than women who follow prescribed guidelines. Reasons for failing to initiate or discontinue hormonal therapy include side effects such as fatigue, hot flashes, vaginal dryness or discharge, and mood swings [6]. However, non-adherence to hormonal therapy recommendations is also associated with factors such as age [10], race, socioeconomic status, and health insurance [7, 10–13].
How health insurance and specifically, prescription drug coverage, influences hormonal therapy adherence is unclear. For example, some studies indicate that Medicaid insurance, which usually has generous prescription drug coverage, increases the likelihood of hormonal therapy initiation [10]; other studies have shown a negative relationship between Medicaid and hormonal therapy adherence [11]. Uninsured women’s patterns of adherence are unknown, although studies of outcomes including recurrence and survival among uninsured women indicate generally unfavorable trends [14]. We hypothesize that differences in hormonal therapy adherence may be at least partially explained by the availability of prescription drug coverage. We focus on prescription drug coverage as opposed to health insurance in general since plans vary in prescription drug benefits. Findings from this study have important implications given the vigorous debates at a national level about health insurance benefits and prescription drug coverage [15] balanced against concerns about drug costs and adherence to potentially life-saving therapies.
Study population
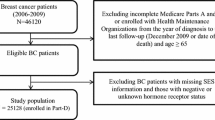
This study was part of a larger study conducted on a longitudinal cohort of women diagnosed with breast cancer in metropolitan Los Angeles and Detroit. Women aged 20–79 years diagnosed with stage 0-III breast cancer between June 2005 and February 2007, as reported to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population-based program registries in those regions were identified and approached for study participation. Methods for sample selection and data collection are published elsewhere [16–18]. Women completed a mailed survey, on average 9 months after diagnosis, and again approximately 4 years later. Responses to the baseline and follow-up surveys were combined into a single dataset, into which clinical data from SEER was merged.
A total of 1536 women were completed both surveys. In order to select a sample appropriate to study hormonal therapy use, we excluded women who were not eligible for hormonal therapy. These women had invasive disease (n = 381), had negative or unknown ER and/or PR receptor status (n = 307), or had experienced a recurrence (n = 41). We also excluded women who had incomplete data on prescription drug coverage (n = 11) and a handful of women with missing data on demographic information (n = 10), leaving 712 women in the analytic sample.
Hormonal therapy and prescription drug coverage
The outcome variables were ‘initiated hormonal therapy’ and ‘hormonal therapy continuation at the second interview.’ Women were considered to have initiated hormonal therapy if they responded affirmatively to either of these follow-up interview questions: “Have you taken any of the following hormonal breast cancer medicines in the past week: Exemestane (Aromasin); Letrozole (Femara);Tamoxifen (Nolvadex); Anastrazole (Arimidex)?” or “Have you ever taken any of the following hormonal breast cancer medicine such as Exemestane (Aromasin), Letrozole (Femara), Tamoxifen (Nolvadex), or Anastrazole (Arimidex)?” Among those women who initiated hormonal therapy, those who responded “yes” to having taken hormonal breast cancer medicines in the past week were considered as ‘continuing hormonal therapy at the second interview.’ We recorded whether patients had prescription drug coverage as a dichotomous variable.
Control variables
Patient demographic variables were also included the analysis. Race/ethnicity was categorized as White, African American, Latina/Spanish-speaking, Latina/English-speaking or other. Age at the time of diagnosis was entered into the model as a continuous variable. We controlled for education (less than a high school degree, high school diploma or GED, some college or technical school, college graduate), marital status (married/partnered or divorced, separated, or widowed, and never married), employment status (working or not working), annual household income (less than $40,000, $40,000 to $69,999, and greater than $70,000 or ‘missing’), cancer stage (I, II, or III). We also included whether women were identified from the Los Angeles or the Detroit SEER registries.
Statistical analysis
The sample characteristics were analyzed descriptively by whether the woman reported having prescription drug coverage at the follow-up survey. Statistically significant differences in the means of continuous normally distributed variables were tested using t tests. Statistical significance of categorical differences (e.g., race, education, marital, employment status) between women with and without prescription drug coverage was determined using Chi-square tests.
We estimated adjusted logistic regression models to measure the relationship between pre-specified independent variables of prescription drug coverage, patient demographic characteristics, cancer stage, and hormonal therapy initiation and continuation. Because having health insurance is positively correlated with having prescription drug coverage, we estimated two sets of models, one that included insurance coverage and another that excluded health insurance coverage. We use self-reported insurance status reported at the first and second interview to create a variable that reflected whether women remained continuously insured (either publicly or privately insured or ‘other’), changed insurance status (gaining insurance, changing from private to public or public to private), or became uninsured and remained so at the time of the second interview. We do not capture periods of uninsurance or a change in a specific policy, but instead have information on the general category of health insurance coverage (public including Medicaid and Medicare, private whether through an employer or purchased policy, a small number of ‘other,’ and uninsured). The estimated odds ratios were virtually unchanged with the addition of these variables (results not shown). Therefore, we report only the results from parsimonious models. We report odds ratios (OR) and 95 % confidence intervals (CI). All analyses were conducted using SAS, version 9.4 and were weighted using survey procedures to account for differential probabilities of sampling and non-response. The study was approved by Institutional Review Boards at the University of Michigan, University of Southern California, and Wayne State University.
Results
Table 1 reports the sample characteristics by whether women had prescription drug coverage. A higher percentage of women with prescription drug coverage initiated and more women continued hormonal therapy at the time of the second interview. Nearly all women with prescription drug coverage initiated hormonal therapy (90 %). At the time of the second survey (approximately 4 years later), 81 % of women with prescription drug coverage were still taking hormonal therapy, whereas only 66 % of the women without prescription drug coverage continued taking hormonal therapy.
Table 2 reports results from adjusted logistic regression models that predict the likelihood of initiating and discontinuing hormonal therapy. Women who had prescription drug coverage were more likely to initiate hormonal therapy relative to women without prescription drug coverage (OR 2.91, 95 % CI 1.24–6.84). Women with prescription drug coverage were also more likely to continue hormonal therapy (OR 2.23; 95 % CI 0.99–5.05, p = 0.0543).
Annual household income was also associated with hormonal therapy initiation. Women with an annual household income below $40,000 were much less likely to initiate hormonal therapy (OR 0.39; 95 % CI 0.18, 0.83). Likewise, the lowest income women were less likely to continue hormonal therapy relative to women with annual household income that equaled or exceeded $70,000 (OR 0.55; 95 % CI 0.29–1.04) with a borderline significance of (p = 0.08).
English-speaking Latinas were more likely to initiate therapy than white patients (OR 2.39, 95 % CI 1.10–5.19). However, this difference was not significant when adjusted for the multiple testing of pairwise comparisons between all racial and ethnic groups.
Conclusions
We found a strong positive relationship between hormonal therapy initiation and prescription drug coverage. A similar relationship was observed for hormonal therapy continuation. Emerging evidence indicates that the out-of-pocket costs of cancer treatment impose a significant burden on cancer patients [19]. A prior published study further suggested that higher prescription drug co-payments are associated with hormonal therapy non-compliance [20]. We add to this literature by jointly examining patient characteristics, health insurance coverage, and prescription drug coverage in a racially diverse sample drawn from a population-based registry. Studies of low-income uninsured and Medicaid insured women are often drawn from teaching hospitals or safety net settings [21], whereas studies of prescription drug coverage are drawn from claims data of privately insured individuals where the effect of having private insurance, which can greatly reduce total financial burden, cannot be disentangled from the prescription drug benefit. The present analysis overcomes these limitations.
The number of oral medications used in oncology is rapidly increasing, making issues regarding adherence, and compliance all the more relevant. This study adds to the body of literature addressing these topics in two ways. Our results demonstrate that prescription drug coverage plays an important role in hormonal therapy initiation and continuation. Moreover, our findings suggest that the prescription drug benefit has an influence on hormonal therapy initiation and continuation, independent of health insurance coverage. Therefore, prescription drug benefits factor into medication adherence, even among insured populations.
Nationally, coverage for prescription medications is the subject of robust debate. The Affordable Care Act (ACA) is gradually lowering the out-of-pocket costs for prescription drugs by offering subsidies to low-income adults and other subsidies that reduce beneficiary coinsurance [22]. Similarly, other ACA provisions such as those to limit cost sharing and out-of-pocket costs may also be important in reducing costs incurred by insured individuals. Given the findings of the current study, these changes may ultimately increase compliance with hormonal therapy recommendations for breast cancer patients.
Our study has a number of strengths including a sample drawn from a population-based registry, inclusion of uninsured women, racial, and ethnic diversity, and its long-term follow-up period. The study has limitations as well. First, the data are self-reported. We did not have information from medical records or prescription drug claims to verify whether women complied with recommendations. Second, we do not have information on the amount of co-payments women were required to pay for hormonal therapy. Third, the study sample, although population-based, was drawn from the Detroit and Los Angeles metropolitan areas and therefore the results may not be generalizable.
In summary, this study demonstrates the critical role of prescription drug coverage in hormonal therapy initiation and continuation, independent of other health insurance coverage. These findings add to the body of literature that addresses medication adherence. Financial factors must be considered along with behavioral factors that influence adherence, which is becoming increasingly relevant to oncology as treatments are shifted to oral medications, many of which are very expensive. The current study suggests that reluctance to insure prescription drugs may result in increased recurrence and poor survival among women with breast cancer, one of the largest groups of cancer survivors.
References
Hershman DL, Greenlee H, Awad D et al (2013) Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat 138(3):795–806
Makubate B, Donnan PT, Dewar JA et al (2013) Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 108(7):151–1524
McCowan C, Shearer J, Donnan PT et al (2008) Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer 99(11):1763–1768
Partridge A, Wang P, Winer E et al (2003) Non-adherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21(4):602–606
Visvanathan K, Hurley P, Bantug E et al (2013) Use of pharmacologic interventions for breast cancer risk reduction: American society of clinical oncology clinical practice guideline. J Clin Oncol 31(23):2942–2962
American Cancer Society (2015) Hormonal therapy for breast cancer. http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-treating-hormone-therapy. Accessed 24 June 2015
Kimmick G, Anderson R, Camacho F et al (2009) Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol 27(21):3445–3451
Partridge AH, LaFountain A, Mayer E et al (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26(4):556–562
Lash TL, Fox MP, Westrup JL et al (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Guy G, Lipscomb J, Gillespie T et al (2014) Variations in guideline-concordant breast cancer adjuvant therapy in rural Georgia. Health Serv Res. doi:10.1111/1475-6773.12269
Wu XC, Lund MJ, Kimmick GG et al (2012) Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol 30(2):142–150
Freedman R, Virgo K, He Y et al (2011) The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer 117(1):180–189
Hershman DL, Tsui J, Wright JD et al (2015) Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol 33(9):1053–1059
Aizer AA, Falit B, Mendu ML et al (2014) Cancer-specific outcomes among young adults without health insurance. J Clin Oncol 32(19):2025–2030
Health and Human Services (2015) Key features of the affordable care act. http://www.hhs.gov/healthcare/facts/timeline/. Accessed 9 July 2015
Jagsi R, Pottow JA, Griffith KA et al (2014) Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol 32(12):1269–1276
Jagsi R, Hawley ST, Abrahamse P et al (2014) Impact of adjuvant chemotherapy on long-term employment of survivors of early-stage breast cancer. Cancer 120(12):1854–1862
Hawley ST, Jagsi R, Morrow M et al (2014) Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. doi:10.1001/jamasurg.2013.5689
Ekwueme DU, Yabroff KR, Guy GP Jr et al (2014) Medical costs and productivity losses of cancer survivors–United States, 2008–2011. MMWR Morb Mortal Wkly Rep 63(23):505–510
Neugut A, Subar M, Wilde ET et al (2011) Association between prescription co-payment amount ad compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol 29(18):2534–2542
Richardson L, Tian L, Voti L et al (2006) The roles of teaching hospitals, insurance status, and race/ethnicity in receipt of adjuvant therapy for regional-stage breast cancer in Florida. Am J Public Health 96(1):160–166
Kaiser Family Foundation (2015). http://kff.org/medicare/fact-sheet/the-medicare-prescription-drug-benefit-fact-sheet/. Accessed 9 July 2015
Acknowledgments
Author contribution
All authors had full access to the data and participated in the design, analysis, and interpretation of the data. Bradley was responsible for drafting the manuscript. All authors reviewed the manuscript before submission.
Funding
This project was funded by Grants R01 CA088370 and R01 CA109696 from the National Cancer Institute to the University of Michigan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any potential conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Bradley, C.J., Dahman, B., Jagsi, R. et al. Prescription drug coverage: implications for hormonal therapy adherence in women diagnosed with breast cancer. Breast Cancer Res Treat 154, 417–422 (2015). https://doi.org/10.1007/s10549-015-3630-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3630-x