Abstract
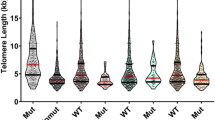
Recently, we observed that telomeres of BRCA1/2 mutation carriers were shorter than those of controls or sporadic breast cancer patients, suggesting that mutations in these genes might be responsible for this event. Given the contradictory results reported in the literature, we tested whether other parameters, such as chemotherapy, could be modifying telomere length (TL). We performed a cross-sectional study measuring leukocyte TL of 266 sporadic breasts cancer patients treated with first-line chemotherapy, with a median follow-up of 240 days. Additionally, we performed both cross-sectional and longitudinal studies in a series of 236 familial breast cancer patients that included affected and non-affected BRCA1/2 mutation carriers. We have measured in leukocytes from peripheral blood: the TL, percentage of short telomeres (<3 kb), telomerase activity levels and the annual telomere shortening speed. In sporadic cases we found that chemotherapy exerts a transient telomere shortening effect (around 2 years) that varies depending on the drug combination. In familial cases, only patients receiving treatment were associated with telomere shortening but they recovered normal TL after a period of 2 years. Chemotherapy affects TL and should be considered in the studies that correlate TL with disease susceptibility.





Similar content being viewed by others
References
Blackburn EH (2001) Switching and signaling at the telomere. Cell 106(6):661–673
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6(8):611–622
Muezzinler A, Zaineddin AK, Brenner H (2013) A systematic review of leukocyte telomere length and age in adults. Ageing Res Rev 12(2):509–519
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345(6274):458–460
Kong CM, Lee XW, Wang X (2013) Telomere shortening in human diseases. FEBS J 280(14):3180–3193
Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomark Prev 20(6):1238–1250
Fyhrquist F, Silventoinen K, Saijonmaa O et al (2011) Telomere length and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. J Hum Hypertens 25(12):711–718
Willeit P, Willeit J, Kloss-Brandstatter A, Kronenberg F, Kiechl S (2011) Fifteen-year follow-up of association between telomere length and incident cancer and cancer mortality. JAMA 306(1):42–44
Willeit P, Willeit J, Mayr A et al (2010) Telomere length and risk of incident cancer and cancer mortality. JAMA 304(1):69–75
Shao L, Wood CG, Zhang D et al (2007) Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol 178(4 Pt 1):1492–1496
Wu X, Amos CI, Zhu Y et al (2003) Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 95(16):1211–1218
Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SE (2013) Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst 105(7):459–468
Savage SA, Gadalla SM, Chanock SJ (2013) The long and short of telomeres and cancer association studies. J Natl Cancer Inst 105(7):448–449
Cunningham JM, Johnson RA, Litzelman K et al (2013) Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomark Prev 22(11):2047–2054
Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E (2011) Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res 39(20):e134
Aviv A, Valdes AM, Spector TD (2006) Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol 35(6):1424–1429
Martinez-Delgado B, Yanowsky K, Inglada-Perez L et al (2011) Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS Genet 7(7):e1002182
Pooley KA, McGuffog L, Barrowdale D et al (2014) Lymphocyte telomere length is longer in BRCA1 and BRCA2 mutation carriers but does not affect subsequent cancer risk. Cancer Epidemiol Biomark Prev 23(6):1018–1024
Killick E, Tymrakiewicz M, Cieza-Borrella C et al (2014) Telomere length shows no association with BRCA1 and BRCA2 mutation status. PLoS One 9(1):e86659
Li P, Hou M, Lou F, Bjorkholm M, Xu D (2012) Telomere dysfunction induced by chemotherapeutic agents and radiation in normal human cells. Int J Biochem Cell Biol 44(9):1531–1540
Liu M, Hales BF, Robaire B (2014) Effects of four chemotherapeutic agents, bleomycin, Etoposide, Cisplatin, and cyclophosphamide, on DNA damage and telomeres in a mouse spermatogonial cell line. Biol Reprod 90(4):72
Milne RL, Osorio A, Cajal TR et al (2008) The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counseling units in Spain. Clin Cancer Res 14(9):2861–2869
Diez O, Osorio A, Duran M et al (2003) Analysis of BRCA1 and BRCA2 genes in Spanish breast/ovarian cancer patients: a high proportion of mutations unique to Spain and evidence of founder effects. Hum Mutat 22(4):301–312
Osorio A, Endt D, Fernandez F et al (2012) Predominance of pathogenic missense variants in the RAD51C gene occurring in breast and ovarian cancer families. Hum Mol Genet 21(13):2889–2898
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30(10):e47
Canela A, Vera E, Klatt P, Blasco MA (2007) High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci USA 104(13):5300–5305
Engelhardt M, Ozkaynak MF, Drullinsky P et al (1998) Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia 12(1):13–24
Schroder CP, Wisman GB, de Jong S et al (2001) Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer 84(10):1348–1353
Sanoff HK, Deal AM, Krishnamurthy J et al (2014) Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst 106(4):dju057
Jacobs JJ, de Lange T (2005) p16INK4a as a second effector of the telomere damage pathway. Cell Cycle 4(10):1364–1368
Diker-Cohen T, Uziel O, Szyper-Kravitz M, Shapira H, Natur A, Lahav M (2013) The effect of chemotherapy on telomere dynamics: clinical results and possible mechanisms. Leuk Lymphoma 54(9):2023–2029
Lu Y, Leong W, Guerin O, Gilson E, Ye J (2013) Telomeric impact of conventional chemotherapy. Front Med 7(4):411–417
Acknowledgments
We thank Alicia Barroso and Victoria Fernandez for their technical support. This work was partially funded by project FIS PI12/00070 from the Carlos III Health Institute and the Spanish Network on Rare Diseases (CIBERER) and by project SAF2012-35779 (Spanish Ministry of Economy and Competiveness). M.A.B.’s Laboratory is funded with the Spanish Ministry of Science and Innovation, Projects SAF2008-05384 and 2007-A-200950 (TELOMARKER), European Research Council Advanced Grant GA#232854, the Körber Foundation, Botín Foundation, and Lilly Foundation.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Standards
The authors declare that this work complies with current Spanish laws.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benitez-Buelga, C., Sanchez-Barroso, L., Gallardo, M. et al. Impact of chemotherapy on telomere length in sporadic and familial breast cancer patients. Breast Cancer Res Treat 149, 385–394 (2015). https://doi.org/10.1007/s10549-014-3246-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3246-6