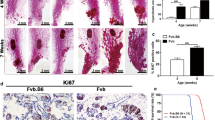
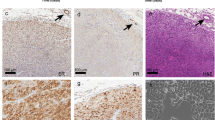
Abstract
Recent studies in patients with breast cancer suggest the immune microenvironment influences response to therapy. We aimed to evaluate the relationship between growth rates of tumors in common spontaneous mammary tumor models and immune biomarkers evaluated in the tumor and blood. TgMMTV-neu and C3(1)-Tag transgenic mice were followed longitudinally from birth, and MPA–DMBA-treated mice from the time of carcinogen administration, for the development of mammary tumors. Tumor-infiltrating CD4+ and CD8+ T-cells, FOXP3+ T-regulatory cells, and myeloid-derived suppressor cells were assessed by flow cytometry. Serum cytokines were evaluated in subsets of mice. Fine needle aspirates of tumors were collected and RNA was isolated to determine levels of immune and proliferation markers. Age of tumor onset and kinetics of tumor growth were significantly different among the models. Mammary tumors from TgMMTV-neu contained a lower CD8/CD4 ratio than that of other models (p < 0.05). MPA–DMBA-induced tumors contained a higher percentage of FOXP3+ CD4+ T-cells (p < 0.01) and MDSC (p < 0.001) compared with the other models. Individuals with significantly slower tumor growth demonstrated higher levels of Type I serum cytokines prior to the development of lesions compared to those with rapid tumor growth. Moreover, the tumors of animals with more rapid tumor growth demonstrated a significant increase in the expression of genes associated with Type II immunity than those with slower-progressing tumors. These data provide a foundation for the development of in vivo models to explore the relationship between endogenous immunity and response to standard therapies for breast cancer.





Similar content being viewed by others
References
Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM (2007) Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 8(5):R76. doi:10.1186/gb-2007-8-5-r76
Usary J, Zhao W, Darr D, Roberts PJ, Liu M, Balletta L, Karginova O, Jordan J, Combest A, Bridges A, Prat A, Cheang MC, Herschkowitz JI, Rosen JM, Zamboni W, Sharpless NE, Perou CM (2013) Predicting drug responsiveness in human cancers using genetically engineered mice. Clin Cancer Res 19(17):4889–4899. doi:10.1158/1078-0432.CCR-13-0522
Shoushtari AN, Michalowska AM, Green JE (2007) Comparing genetically engineered mouse mammary cancer models with human breast cancer by expression profiling. Breast Dis 28:39–51
Vargo-Gogola T, Rosen JM (2007) Modelling breast cancer: one size does not fit all. Nat Rev Cancer 7(9):659–672. doi:10.1038/nrc2193
Van Dyke T, Jacks T (2002) Cancer modeling in the modern era: progress and challenges. Cell 108(2):135–144
Lanari C, Lamb CA, Fabris VT, Helguero LA, Soldati R, Bottino MC, Giulianelli S, Cerliani JP, Wargon V, Molinolo A (2009) The MPA mouse breast cancer model: evidence for a role of progesterone receptors in breast cancer. Endocr Relat Cancer 16(2):333–350. doi:10.1677/ERC-08-0244
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ (1992) Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA 89(22):10578–10582
Maroulakou IG, Anver M, Garrett L, Green JE (1994) Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci USA 91(23):11236–11240
Aldaz CM, Liao QY, LaBate M, Johnston DA (1996) Medroxyprogesterone acetate accelerates the development and increases the incidence of mouse mammary tumors induced by dimethylbenzanthracene. Carcinogenesis 17(9):2069–2072
Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29(1):52–54
Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE (2000) The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19(8):968–988
Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, Azeke E, Disis ML (2006) IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J Immunol 177(1):84–91
Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, Fintak P, Childs J, Dela Rosa C, Disis ML (2008) Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res 68(20):8400–8409. doi:10.1158/0008-5472.CAN-07-5891
Mao J, Ladd J, Gad E, Rastetter L, Johnson MM, Marzbani E, Childs JS, Lu H, Dang Y, Broussard E, Stanton SE, Hanash SM, Disis ML (2014) Mining the pre-diagnostic antibody repertoire of TgMMTV-neu mice to identify autoantibodies useful for the early detection of human breast cancer. J Transl Med 12:121. doi:10.1186/1479-5876-12-121
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61(12):4766–4772
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10(9):942–949. doi:10.1038/nm1093
Wang RF, Peng G, Wang HY (2006) Regulatory T cells and toll-like receptors in tumor immunity. Semin Immunol 18(2):136–142. doi:10.1016/j.smim.2006.01.008
Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59(10):1593–1600. doi:10.1007/s00262-010-0855-8
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892. doi:10.1056/NEJMoa1113205
Andrechek ER, Cardiff RD, Chang JT, Gatza ML, Acharya CR, Potti A, Nevins JR (2009) Genetic heterogeneity of Myc-induced mammary tumors reflecting diverse phenotypes including metastatic potential. Proc Natl Acad Sci USA 106(38):16387–16392. doi:10.1073/pnas.0901250106
Franci C, Zhou J, Jiang Z, Modrusan Z, Good Z, Jackson E, Kouros-Mehr H (2013) Biomarkers of residual disease, disseminated tumor cells, and metastases in the MMTV-PyMT breast cancer model. PLoS ONE 8(3):e58183. doi:10.1371/journal.pone.0058183
Lu H, Knutson KL, Gad E, Disis ML (2006) The tumor antigen repertoire identified in tumor-bearing neu transgenic mice predicts human tumor antigens. Cancer Res 66(19):9754–9761. doi:10.1158/0008-5472.CAN-06-1083
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113. doi:10.1200/JCO.2009.23.7370
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24(34):5373–5380. doi:10.1200/JCO.2006.05.9584
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 31(7):860–867. doi:10.1200/JCO.2011.41.0902
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29(15):1949–1955. doi:10.1200/JCO.2010.30.5037
Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD (2011) Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology 58(7):1107–1116. doi:10.1111/j.1365-2559.2011.03846.x
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO (2012) CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast cancer Res BCR 14(2):R48. doi:10.1186/bcr3148
Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G (2013) Tumor-associated lymphocytes predict response to neo adjuvant chemotherapy in breast cancer patients. J Breast Cancer 16(1):32–39. doi:10.4048/jbc.2013.16.1.32
Acknowledgments
The authors wish to thank the staff of the University of Washington Comparative Pathology Program/Histology and Imaging Core Research Laboratory especially Brian Johnson, Erin McCarty, and Cara Appel for their contributions to slide production as well as histochemical and immunohistochemical staining. This work was supported by the National Cancer Institute, U01 CA141539, the Department of Defense Grant, W81XWH-11-1-0760, and the National Cancer Institute contract, N01-CN-53300/WA#10. Mary L. Disis was supported by The Athena Distinguished Professorship for Breast Cancer Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All experiments performed for this manuscript comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ekram Gad and Lauren Rastetter have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gad, E., Rastetter, L., Slota, M. et al. Natural history of tumor growth and immune modulation in common spontaneous murine mammary tumor models. Breast Cancer Res Treat 148, 501–510 (2014). https://doi.org/10.1007/s10549-014-3199-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3199-9