Abstract
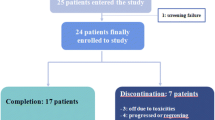
Neoadjuvant endocrine therapy is an alternative to chemotherapy for women with oestrogen receptor (ER)-positive early breast cancer (BC). We aimed to assess feasibility of recruiting patients to a study comparing chemotherapy versus endocrine therapy in postmenopausal women with ER-rich primary BC, and response as well as translational endpoints were assessed. Patients requiring neoadjuvant therapy were randomised to chemotherapy: 6 × 3-weekly cycles FE100C or endocrine therapy: letrozole 2.5 mg, daily for 18–23 weeks. Primary endpoints were recruitment feasibility and tissue collection. Secondary endpoints included clinical, radiological and pathological response rates, quality of life and translational endpoints. 63/80 patients approached were eligible, of those 44 (70, 95 % CI 57–81) were randomised. 12 (54.5, 95 % CI 32.2–75.6) chemotherapy patients showed radiological objective response compared with 13 (59.1, 95 % CI 36.4–79.3) letrozole patients. Compared with baseline, mean Ki-67 levels fell in both groups at days 2–4 and at surgery [fold change: 0.24 (95 % CI 0.12–0.51) and 0.24; (95 % CI 0.15–0.37), respectively]. Plasma total cfDNA levels rose from baseline to week 8 [fold change: chemotherapy 2.10 (95 % CI 1.47–3.00), letrozole 1.47(95 % CI 0.98–2.20)], and were maintained at surgery in the chemotherapy group [chemotherapy 2.63; 95 % CI 1.56–4.41), letrozole 0.95 (95 % CI 0.71–1.26)]. An increase in plasma let-7a miRNA was seen at surgery for patients with objective radiological response to chemotherapy. Recruitment and tissue collection endpoints were met; however, a larger trial was deemed unfeasible due to slow accrual. Both regimens were equally efficacious. Dynamic changes were seen in Ki-67 and circulating biomarkers in both groups with increases in cfDNA and let-7a miRNA persisting until surgery for chemotherapy patients.




Similar content being viewed by others
References
Kaufmann M, von Minckwitz G, Smith R et al (2003) International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol 21:2600–2608
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97:188–194
Colleoni M, Viale G, Zahrieh D et al (2004) Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res 10:6622–6628
Gianni L, Pienkowski T, Im YH et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32
Baselga J, Bradbury I, Eidtmann H et al (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379:633–640
Aebi S, Sun Z, Braun D et al (2011) Differential efficacy of three cycles of CMF followed by tamoxifen in patients with ER-positive and ER-negative tumors: long-term follow up on IBCSG Trial IX. Ann Oncol 22:1981–1987
Viale G, Regan MM, Maiorano E et al (2008) Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: predictive value of centrally reviewed expression of estrogen and progesterone receptors–International Breast Cancer Study Group. J Clin Oncol 26:1404–1410
Pagani O, Gelber S, Simoncini E et al (2009) Is adjuvant chemotherapy of benefit for postmenopausal women who receive endocrine treatment for highly endocrine-responsive, node-positive breast cancer? International Breast Cancer Study Group Trials VII and 12-93. Breast Cancer Res Treat 116:491–500
Berry DA, Cirrincione C, Henderson IC et al (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667
Palmieri C, Patten DK, Januszewski A et al (2014) Breast cancer: current and future endocrine therapies. Mol Cell Endocrinol 382:695–723
Eiermann W, Paepke S, Appfelstaedt J et al (2001) Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol 12:1527–1532
Cataliotti L, Buzdar AU, Noguchi S et al (2006) Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer 106:2095–2103
Krainick-Strobel UE, Lichtenegger W, Wallwiener D et al (2008) Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer 8:62
Early Breast Cancer Trialists’ Collaborative G, Davies C, Godwin J et al.(2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784
Ellis MJ, Coop A, Singh B et al (2001) Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 19:3808–3816
Clarke RB, Laidlaw IJ, Jones LJ et al (1993) Effect of tamoxifen on Ki67 labelling index in human breast tumours and its relationship to oestrogen and progesterone receptor status. Br J Cancer 67:606–611
Dowsett M, Ebbs SR, Dixon JM et al (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol 23:2477–2492
Dowsett M, Smith IE, Ebbs SR et al (2007) Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170
Ellis MJ, Tao Y, Luo J et al (2008) Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 100:1380–1388
Semiglazov VF, Semiglazov VV, Dashyan GA et al (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110:244–254
Shaw JA, Smith BM, Walsh T et al (2000) Microsatellite alterations plasma DNA of primary breast cancer patients. Clin Cancer Res 6:1119–1124
Hoque MO, Feng Q, Toure P et al (2006) Detection of aberrant methylation of four genes in plasma DNA for the detection of breast cancer. J Clin Oncol 24:4262–4269
Beaver JA, Jelovac D, Balukrishna S et al (2014) Detection of cancer DNA in plasma of early stage breast cancer patients. Clin Cancer Res 20(10):2643–2650
Mitchell PS, Parkin RK, Kroh EM et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105:10513–10518
Chen X, Ba Y, Ma L et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006
Yamamoto Y, Kosaka N, Tanaka M et al (2009) MicroRNA-500 as a potential diagnostic marker for hepatocellular carcinoma. Biomarkers 14:529–538
Jung K, Fleischhacker M, Rabien A (2010) Cell-free DNA in the blood as a solid tumor biomarker–a critical appraisal of the literature. Clin Chim Acta 411:1611–1624
Shaw JA, Page K, Blighe K et al (2012) Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res 22:220–231
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403(6772):901–906
Zhao Y, Deng C, Wang J, Xiao J, Gatalica Z, Recker RR, Xiao GG (2011) Let-7 family miRNAs regulate estrogen receptor alpha signaling in estrogen receptor positive breast cancer. Breast Cancer Res Treat 127:69–80
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E (2007) let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131:1109–1123
Sun X, Qin S, Fan C et al (2013) Let-7: a regulator of the ERalpha signaling pathway in human breast tumors and breast cancer stem cells. Oncol Rep 29:2079–2087
Semiglazov VF, Semiglazov VV, Dashyan GA et al (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110:244–254
Alba E, Calvo L, Albanell J et al (2012) Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol 23:3069–3074
Acknowledgments
We thank the women who took part in this study; the doctors, nurses and support staff at the following local sites: Asan Medical Centre (27 patients), Charing Cross Hospital (3 patients), St Mary’s Hospital (3 patients), Bristol Haematology and Oncology Centre(2 patients), Guys and St Thomas (2 patients), Aberdeen Royal Infirmary (1 patient), Ninewells Hospital and Medical School, Dundee (4 patients), West Middlesex University Hospital (1 patient), Royal Alexandra Hospital (1 patient). The following sites were open but did not recruit any patients, The Beatson West of Scotland Cancer Centre, Southampton University Hospitals Trust, Crosshouse and Ayr Hospitals and Inverclyde Hospital. We also thank the members of the study steering committee and the independent data monitoring committee. NEOCENT was an NIHR Clinical Research Network portfolio trial, and we acknowledge the help of the local research networks that supported recruitment at UK sites. The study was supported by Cancer Research UK through CTAAC award (Grant No. C37/A9356), a Clinical and Translational Research Committee Programme Award (Grant No. C14315/A13462), and the ECMC network as well as an educational grant from Novartis. Carlo Palmieri was a recipient of a Cancer Research UK Clinician Scientist Award and Basma Rghebi is supported by the Lybian Government. The ICR-CTSU also receives core funding from Cancer Research UK. Dundee recruitment was supported by the Grant Simpson Trust. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
S.B. Kim received research funding from Novartis unrelated to this study. Remaining authors have no conflicts of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the NEOCENT Trial Investigators.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palmieri, C., Cleator, S., Kilburn, L.S. et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat 148, 581–590 (2014). https://doi.org/10.1007/s10549-014-3183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3183-4