Abstract
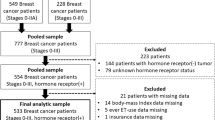
Adjuvant endocrine therapy for breast cancer reduces recurrence and improves survival rates. Many patients never start treatment or discontinue prematurely. A better understanding of factors associated with endocrine therapy initiation and persistence could inform practitioners how to support patients. We analyzed data from a longitudinal study of 2,268 women diagnosed with breast cancer and reported to the Metropolitan Detroit and Los Angeles SEER cancer registries in 2005–2007. Patients were surveyed approximately both 9 months and 4 years after diagnosis. At the 4-year mark, patients were asked if they had initiated endocrine therapy, terminated therapy, or were currently taking therapy (defined as persistence). Multivariable logistic regression models examined factors associated with initiation and persistence. Of the 743 patients eligible for endocrine therapy, 80 (10.8 %) never initiated therapy, 112 (15.1 %) started therapy but discontinued prematurely, and 551 (74.2 %) continued use at the second time point. Compared with whites, Latinas (OR 2.80, 95 % CI 1.08–7.23) and black women (OR 3.63, 95 % CI 1.22–10.78) were more likely to initiate therapy. Other factors associated with initiation included worry about recurrence (OR 3.54, 95 % CI 1.31–9.56) and inadequate information about side effects (OR 0.24, 95 % CI 0.10–0.55). Factors associated with persistence included two or more medications taken weekly (OR 4.19, 95 % CI 2.28–7.68) and increased age (OR 0.98, 95 % CI 0.95–0.99). Enhanced patient education about potential side effects and the effectiveness of adjuvant endocrine therapy in improving outcomes may improve initiation and persistence rates and optimize breast cancer survival.

Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9:45–53
Yood MU, Owusu C, Buist DS et al (2008) Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg 206:66–75
Swedish Breast Cancer Cooperative Group (1996) Randomized trial of two versus 5 years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 88:1543–1549
Sacco M, Valentini M, Belfiglio M et al (2003) Randomized trial of 2 versus 5 years of adjuvant tamoxifen for women aged 50 years or older with early breast cancer: Italian Interdisciplinary Group Cancer Evaluation Study of Adjuvant Treatment in Breast Cancer 01. J Clin Oncol 21:2276–2281
Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol 2012. doi:10.1097/COC.0b013e3182436ec1 Accessed 29 Sept 2012
Hershman DL, Shao T, Kushi LH et al (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126:529–537
Partridge AH, Lafountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21:602–606
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 3.2012. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 1 Oct 2012
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2003) Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol 21:3357–3365
Thuerlimann B (2001) International consensus meeting on the treatment of primary breast cancer 2001, St. Gallen, Switzerland. Breast Cancer 8:294–297
Davies C, Hongchao P, Godwin J, et al (2012) ATLAS. 10 v 5 years of adjuvant tamoxifen (TAM) in ER+ disease: effects on outcome in the first and in the second decade after diagnosis. CTRC–AACR San Antonio Breast Cancer Symposium, San Antonio
Jin H, Tu D, Zhao N et al (2012) Longer-term outcomes of Letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 30(7):718–721
Buzdar A, Howell A, Cuzick J et al (2006) Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol 7:633–643
Henry NL, Giles JT, Ang D et al (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111:365–372
Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J (2002) Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst 94:1626–1634
Harlan LC, Clegg LX, Abrams J, Stevens JL, Ballard-Barbash R (2006) Community-based use of chemotherapy and hormonal therapy for early-stage breast cancer: 1987–2000. J Clin Oncol 24:872–877
Hebert-Croteau N, Brisson J, Latreille J, Gariepy G, Blanchette C, Deschenes L (1999) Time trends in systemic adjuvant treatment for node-negative breast cancer. J Clin Oncol 17:1458–1464
Lash TL, Silliman RA, Guadagnoli E, Mor V (2000) The effect of less than definitive care on breast carcinoma recurrence and mortality. Cancer 89:1739–1747
Atkins L, Fallowfield L (2006) Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer 42:2271–2276
Demissie S, Silliman RA, Lash TL (2001) Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol 19:322–328
Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA (2004) Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol 22:3309–3315
Grunfeld EA, Hunter MS, Sikka P, Mittal S (2005) Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns 59:97–102
Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM (2007) Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care 45:431–439
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Chlebowski RT, Geller ML (2006) Adherence to endocrine therapy for breast cancer. Oncology. 71:1–9
DiMatteo MR (2004) Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 42:200–209
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Owusu C, Buist DS, Field TS et al (2008) Predictors of tamoxifen discontinuation among older women with estrogen receptor: positive breast cancer. J Clin Oncol 26:549–555
Doshi JA, Zhu J, Lee BY, Kimmel SE, Volpp KG (2009) Impact of a prescription copayment increase on lipid-lowering medication adherence in veterans. Circulation 119:390–397
Piette JD, Heisler M (2004) Problems due to medication costs among VA and non-VA patients with chronic illnesses. Am J Manag Care 10:861–868
Piette JD, Heisler M, Krein S, Kerr EA (2005) The role of patient-physician trust in moderating medication nonadherence due to cost pressures. Arch Intern Med 165:1749–1755
Griggs JJ, Hawley ST, Graff JJ et al (2012) Factors associated with receipt of breast cancer adjuvant chemotherapy in a diverse population-based sample. J Clin Oncol 30:3058–3064
Dillman DA (2007) Mail and Internet Surveys: the tailored design method, 2nd edn. Wiley, New York
Hamilton AS, Hofer TP, Hawley ST et al (2009) Latinas and breast cancer outcomes: population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev 18:2022–2029
Horne R, Weinman J (1999) Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 47:555–567
Janz NK, Hawley ST, Mujahid MS et al (2011) Correlates of worry about recurrence in a multiethnic population-based sample of women with breast cancer. Cancer 117:1827–1836
Danilak M, Chambers CR (2012) Adherence to adjuvant endocrine therapy in women with breast cancer. J Oncol Pharm Pract Aug 15 (epub ahead of print)
Yen TW, Czypinski LK, Sparapani RA et al (2011) Socioeconomic factors associated with adjuvant hormone therapy use in older breast cancer survivors. Cancer 117:398–405
Yung RL, Hassett MJ, Chen K (2012) et al. Initiation of adjuvant hormone therapy by Medicaid insured women with nonmetastatic breast cancer. J Natl Cancer Inst 104:1102–1105
Lipscomb J, Gillespie TW, Goodman M et al (2012) Black-white differences in receipt and completion of adjuvant chemotherapy among breast cancer patients in a rural region of the US. Breast Cancer Res Treat 133:285–296
Kimmick GG, Anderson R, Camacho F et al (2009) Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol 27:3445–3551
Land SR, Cronin WM, Wickerham DL et al (2011) Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the national surgical adjuvant breast and bowel project p-1 breast cancer prevention trial. Cancer Prev Res 4:1393–1400
Lin JH, Zhang SM, Manson JE (2011) Predicting adherence to tamoxifen and breast cancer adjuvant therapy and prevention. Cancer Prev Res. 4:1360–1365
Friese CR (2012) Disparities in breast cancer care delivery: solving a complex puzzle. Breast Cancer Res Treat 133:297–299
Janz NK, Becker MH (1984) The health belief model: a decade later. Health Educ Q 11:1–47
Wu XC, Lund MJ, Kimmick GG et al (2011) Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for loco regional breast cancers. J Clin Oncol 30(2):142–150
Oberguggenberger A, Sztankay M, Beer B, et al (2012) Adherence evaluation of endocrine treatment in breast cancer: methodological aspects. BMC Cancer 12:474. http://www.biomedcentral.com/1471-2407/12/474 Accessed on 22 Oct 2012
Acknowledgments
This work was funded by Grants R01 CA109696 and R01 CA088370 from the National Cancer Institute to the University of Michigan. Dr. Friese was supported by a Pathway to Independence Award from the National Institute for Nursing Research (R00NR01570). Dr. Katz was supported by an Established Investigator Award in Cancer Prevention, Control, Behavioral, and Population Sciences Research from the National Cancer Institute (K05CA111340). Dr. Jagsi was supported by a Mentored Research Scholar Grant from the American Cancer Society (MRSG-09-145-01). The collection of Los Angeles County cancer incidence data was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code §103885. The National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract N01-PC-35139 was awarded to the University of Southern California. Contract N01-PC-54404 and agreement 1U58DP00807-01 were awarded to the Public Health Institute. The collection of metropolitan Detroit cancer incidence data was supported by the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract N01-PC-35145.
Conflict of interest
The authors declare they have no conflict of interest.
Ethical standards
Institutional Review Boards of the University of Michigan, the University of Southern California, and Wayne State University approved the study described in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friese, C.R., Pini, T.M., Li, Y. et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat 138, 931–939 (2013). https://doi.org/10.1007/s10549-013-2499-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2499-9