Abstract
Background
Breast cancer incidence rates vary according to estrogen receptor expression (ER) and histopathology. We hypothesized that annual mortality rates from breast cancer after initial diagnosis (hazard rates) might also vary by ER and histopathology.
Methods
We accessioned the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER, 1992–2002) program to estimate hazard rates according to ER (positive and negative) and histopathology (duct, tubular, lobular, medullary, inflammatory, papillary, and mucinous types). We used spline functions to model hazard rates free of strongly parametric assumptions for ER negative and positive cases overall and by histopathology.
Results
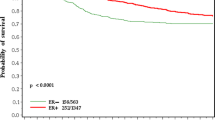
Hazard rates for ER negative and ER positive cases were distinct and non-proportional. At 17 months, ER negative hazard rates peaked at 7.5% per year (95% CI, 7.3–7.8% per year) then declined, whereas ER positive hazard rates lacked a sharp early peak and were comparatively constant at 1.5–2% per year. Falling ER negative and constant ER positive hazard rates crossed at 7 years; after which, prognosis was better for ER negative cases. Among ER positive and negative cases, there were proportional and non-proportional hazards according to histopathologic type, but the two basic ER-associated patterns were maintained.
Conclusions
Hazard rates differed quantitatively and qualitatively according to ER and histopathology. These large-scale population-based results seem consistent with genomic studies, demonstrating two main classes of breast cancers with distinct prognoses according to ER expression.
Similar content being viewed by others
References
Brinkley D, Haybittle J (1984) Long-term survival of women with breast cancer. Lancet 1:1118
Gore SM, Pocock SJ, Kerr GR (1984) Regression models and non-proportional hazards in the analysis of breast cancer survival. Appl Stat 33:176–195
Saphner T, Tormey DC, Gray R (1996) Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 14:2738–2746
Yakovlev AY, Tsodikov AD, Boucher K, Kerber R (1999) The shape of the hazard function in breast carcinoma: curability of the disease revisited. Cancer 85:1789–1798
Gray RJ (1994) Spline-based tests in survival analysis. Biometrics 50:640–652
Gray RJ (1992) Flexible methods for analyzing survival data using splines, with application to breast cancer prognosis. J Am Stat Assoc 87:942–951
Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM (1998) Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat 52:227–237
Demicheli R, Valagussa P, Bonadonna G (2002) Double-peaked time distribution of mortality for breast cancer patients undergoing mastectomy. Breast Cancer Res Treat 75:127–134
Boracchi P, Biganzoli E, Marubini E (2003) Joint modelling of cause-specific hazard functions with cubic splines: an application to a large series of breast cancer patients. Comput Stat Data Anal 42:243–262
Jatoi I, Tsimelzon A, Weiss H, Clark GM, Hilsenbeck SG (2005) Hazard rates of recurrence following diagnosis of primary breast cancer. Breast Cancer Res Treat 89:173–178
Anderson WF, Jatoi I, Devesa SS (2005) Distinct breast cancer incidence and prognostic patterns in the NCI’s SEER program: suggesting a possible link between etiology and outcome. Breast Cancer Res Treat 90:127–137
Baum M, Demicheli R, Hrushesky W, Retsky M (2005) Does surgery unfavourably perturb the “natural history” of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer 41:508–515
Takeuchi H, Baba H, Kano T, Maehara Y (2005) The time-related changes of the importance of prognostic factors in breast cancer. A sequential multivariate analysis of 1423 Japanese patients. Breast Cancer Res Treat 94:273–278
SEER: Surveillance, Epidemiology, and End Results (SEER) Program. Public-Use Database (1973–2002), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2005, based on the November 2004 submission, 2005, www.seer.cancer.gov
Berg JW, Hutter RV (1995) Breast cancer. Cancer 75:257–269
SEER: ICD-O-3 Coding Materials, 2004, http://seer.cancer.gov/icd-o-3/
Rosenberg PS (1995) Hazard function estimation using B-splines. Biometrics 51:874–887
Akaike H (1973) In: Petrov BN, Csaki F (eds) 2nd International symposium on information theory, Budapest, Akademiai Kiado, pp 267–281
Efron B (1982) The jackknife, the bootstrap, and other resampling plans. Society for Industrial and Applied Mathematics, Philadelphia
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman and Hall, New York
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Method) 57:289–300
Cox DR (1972) Regression models and life-tables. J Roy Stat Soc B 34:187–220
Anderson WF, Chu KC, Chang S, Sherman ME (2004) Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev 13:1128–1135
Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J (2002) Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst 94:1626–1634
Chu KC, Tarone RE, Kessler LG, Ries LAG, Hankey BF, Miller BA, Edwards BK (1996) Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst 88:1571–1579
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717
Spratt JS Jr, Kaltenbach ML, Spratt JA (1977) Cytokinetic definition of acute and chronic breast cancer. Cancer Res 37:226– 230
Lilienfeld AM, Johnson EA (1955) The age distribution in female breast and genital cancers. Cancer 8:875–882
De Waard F, De Laive JWJ, Baanders-van Halewijn EA (1960) On bimodal age distribution of mammary carcinoma. Br J Cancer 14:437–448
Rosen PP, Oberman HA (1993) Tumors of the mammary gland. Armed Forces Institue of Pathology, Washington, DC
Anderson WF, Althuis MD, Brinton LA, Devesa SS (2004) Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat 83:77–86
Anderson WF, Chu KC, Devesa SS (2004) Distinct incidence patterns among in-situ and invasive breast carcinomas, with possible etiologic implications. Breast Cancer Res Treat 88:149–159
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Winer EP, Carey LA, Dowsett M, Tripathy D (2005) In: Michael C Perry (ed) American society of clinical onocolgy education book. American Society of Clinical Oncology, Alexandria, VA, pp 46–59
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH/National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, W.F., Chen, B.E., Jatoi, I. et al. Effects of Estrogen Receptor Expression and Histopathology on Annual Hazard Rates of Death from Breast Cancer. Breast Cancer Res Treat 100, 121–126 (2006). https://doi.org/10.1007/s10549-006-9231-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9231-y