Abstract
The neurons of the central nervous system (CNS) require precise control of their bathing microenvironment for optimal function, and an important element in this control is the blood–brain barrier (BBB). The BBB is formed by the endothelial cells lining the brain microvessels, under the inductive influence of neighbouring cell types within the ‘neurovascular unit’ (NVU) including astrocytes and pericytes. The endothelium forms the major interface between the blood and the CNS, and by a combination of low passive permeability and presence of specific transport systems, enzymes and receptors regulates molecular and cellular traffic across the barrier layer. A number of methods and models are available for examining BBB permeation in vivo and in vitro, and can give valuable information on the mechanisms by which therapeutic agents and constructs permeate, ways to optimize permeation, and implications for drug discovery, delivery and toxicity. For treating lysosomal storage diseases (LSDs), models can be included that mimic aspects of the disease, including genetically-modified animals, and in vitro models can be used to examine the effects of cells of the NVU on the BBB under pathological conditions. For testing CNS drug delivery, several in vitro models now provide reliable prediction of penetration of drugs including large molecules and artificial constructs with promising potential in treating LSDs. For many of these diseases it is still not clear how best to deliver appropriate drugs to the CNS, and a concerted approach using a variety of models and methods can give critical insights and indicate practical solutions.



Similar content being viewed by others
Abbreviations
- AAV:
-
adeno-associated virus
- ABC:
-
ATP-binding cassette
- ABCG2:
-
BCRP
- ADME:
-
absorption, distribution, metabolism, excretion
- AMT:
-
adsorptive-mediated transcytosis
- BBB:
-
blood–brain barrier
- BBEC:
-
bovine brain endothelial cells (primary)
- BCRP:
-
breast cancer resistance protein
- bEND3:
-
mouse immortalised brain endothelial cell line
- CNS:
-
central nervous system
- CSF:
-
cerebrospinal fluid
- CYP:
-
cytochrome P450 enzyme
- CVO:
-
circumventricular organ
- ERT:
-
enzyme replacement therapy
- GAGs:
-
glycosaminoglycans
- GLUT1:
-
glucose carrier
- hCMEC/D3:
-
human immortalised brain endothelial cell line
- hPSCs:
-
human pluripotent stem cells
- IL-1, IL17A:
-
interleukins
- INCL:
-
infantile neuronal ceroid lipofuscinosis
- IV:
-
intravenous
- K p,uu :
-
unbound drug brain:plasma concentration ratio
- LAT1:
-
large neutral amino acid carrier
- LDL:
-
low density lipoprotein
- LogBB (or Kp):
-
total drug brain:plasma concentration ratio
- LogD octanol :
-
log compound distribution coefficient octanol/buffer at given pH
- LogP octanol :
-
log compound partition coefficient octanol/water, neutral species
- LSD:
-
lysosomal storage disease
- MPR:
-
mannose-6-phosphate receptor
- MDR1 (or PgP):
-
P-glycoprotein
- MMP:
-
matrix metalloproteinase
- MPS:
-
mucopolysaccharidosis
- MPSIIIA or B:
-
Sanfilippo syndrome type A or B
- MPSVII:
-
Gaucher’s disease
- miRNA:
-
microRNA
- NVU:
-
neurovascular unit
- PAMPA:
-
parallel artificial membrane permeability assay
- P app :
-
apparent permeability
- PBEC:
-
porcine brain endothelial cells (primary)
- PBEC/As:
-
PBEC co-cultured with rat astrocytes
- PDGF-B:
-
platelet-derived growth factor B
- P e :
-
endothelial permeability
- PgP:
-
P-glycoprotein (or MDR1, ABCB1)
- PS :
-
permeability x surface area product
- QSAR:
-
quantitative structure-activity relationship
- RBEC:
-
rat brain endothelial cells (primary)
- RBEC/As:
-
RBEC co-cultured with rat astrocytes
- RMT:
-
receptor-mediated transcytosis
- SAR:
-
structure-activity relationship
- SLC:
-
small solute carrier
- SRT:
-
substrate-replacement therapy
- TEER:
-
transendothelial electrical resistance
- XMET:
-
xenobiotic metabolising enzymes and transporters
References
Abbott NJ (1992) Comparative physiology of the blood–brain barrier. In: Bradbury MWB (ed) Physiology and pharmacology of the blood–brain barrier (Handbk Exp Pharmacol 103). Springer, Heidelberg, pp 371–396
Abbott NJ (2004a) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552
Abbott NJ (2004b) Prediction of blood–brain barrier permeation in drug discovery, from in vivo, in vitro and in silico models. Drug Discov Today: Technol 1:407–416
Abbott NJ, Friedman A (2012) Overview and introduction: the blood–brain barrier in health and disease. Epilepsia 53(Suppl 6:1–6)
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7:41–53
Abbott NJ, Dolman DE, Patabendige AK (2008) Assays to predict drug permeation across the blood–brain barrier, and distribution to brain. Curr Drug Metab 9:901–910
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Anson DS, McIntyre C, Byers S (2011) Therapies for neurological disease in the mucopolysaccharidoses. Curr Gene Ther 11:132–143
Arfi A, Richard M, Gandolphe C, Bonnefont-Rousselot D, Thérond P, Scherman D (2011) Neuroinflammatory and oxidative stress phenomena in MPS IIIA mouse model: the positive effect of long-term aspirin treatment. Mol Genet Metab 103:18–25
Armulik A, Genové G, Mäe M et al (2010) Pericytes regulate the blood–brain barrier. Nature 468:557–561
Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215
Avdeef A (2011) How well can in vitro brain microcapillary endothelial cell models predict rodent in vivo blood–brain barrier permeability? Eur J Pharm Sci 43:109–124
Avdeef A (2012) Absorption and drug development: Solubility, permeability, and charge, 2nd edn. Wiley, Hoboken, p 698pp
Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood–brain barrier. Progr Drug Res 61:39–78
Begley DJ, Pontikis CC, Scarpa M (2008) Lysosomal storage diseases and the blood–brain barrier. Curr Pharm Des 14:1566–1580
Bell RD, Winkler EA, Sagare AP et al (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427
Bellettato CM, Scarpa M (2010) Pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis 33:347–362
Benarroch EE (2011) Circumventricular organs: receptive and homeostatic functions and clinical implications. Neurology 77:1198–1204
Bikadi Z, Hazai I, Malik D et al (2011) Predicting P-glycoprotein-mediated drug transport based on support vector machine and three-dimensional crystal structure of P-glycoprotein. PLoS One 6:e25815
Cabrera-Salazar MA, Deriso M, Bercury SD et al (2012) Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease. PLoS One 7:e43310
Calias P, Papisov M, Pan J et al (2012) CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS One 7:e30341
Candela P, Gosselet F, Miller F et al (2008) Physiological pathway for low-density lipoproteins across the blood–brain barrier: transcytosis through brain capillary endothelial cells in vitro. Endothelium 15:254–264
Candela P, Gosselet F, Saint-Pol J et al (2010) Apical-to-basolateral transport of amyloid-β peptides through blood–brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimers Dis 22:849–859
Carruthers A, DeZutter J, Ganguly A, Devaskar SU (2009) Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab 297:E836–E848
Cecchelli R, Dehouck B, Descamps L et al (1999) In vitro model for evaluating drug transport across the blood–brain barrier. Adv Drug Deliv Rev 36:165–178
Cecchelli R, Berezowski V, Lundquist S et al (2007) Modelling of the blood–brain barrier in drug discovery and development. Nat Rev Drug Discov 6:650–661
Chen YH, Claflin K, Geoghegan JC, Davidson BL (2012) Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol Ther 20:1393–1399
Costantino L, Boraschi D (2012) Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today 17:367–378
Dagenais C, Avdeef A, Tsinman O, Dudley A, Beliveau R (2009) P-glycoprotein deficient mouse in situ blood–brain barrier permeability and its prediction using an in combo PAMPA model. Eur J Pharm Sci 38:121–137
Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA (2009) Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 106:641–646
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature 468:562–566
Dauchy S, Dutheil F, Weaver RJ et al (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood–brain barrier. J Neurochem 107:1518–1528
Deli MA, Abrahám CS, Kataoka Y, Niwa M (2005) Permeability studies on in vitro blood–brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol 25:59–127
Descamps L, Dehouck MP, Torpier G, Cecchelli R (1996) Receptor-mediated transcytosis of transferrin through blood–brain barrier endothelial cells. Am J Physiol 270:H1149–H1158
Dickson PI, Chen AH (2011) Intrathecal enzyme replacement therapy for mucopolysaccharidosis I: translating success in animal models to patients. Curr Pharm Biotechnol 12:946–955
Dickson P, McEntee M, Vogler C et al (2007) Intrathecal enzyme replacement therapy: successful treatment of brain disease via the cerebrospinal fluid. Mol Genet Metab 91:61–68
Dohgu S, Ryerse JS, Robinson SM, Banks WA (2012) Human immunodeficiency virus-1 uses the mannose-6-phosphate receptor to cross the blood–brain barrier. PLoS One 7:e39565
Dutheil F, Jacob A, Dauchy S (2010) ABC transporters and cytochromes P450 in the human central nervous system: influence on brain pharmacokinetics and contribution to neurodegenerative disorders. Expert Opin Drug Metab Toxicol 6:1161–1174
Engelhardt B, Ransohoff RM (2012) Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol 33:579–589
Farfel-Becker T, Vitner EB, Pressey SN, Eilam R, Cooper JD, Futerman AH (2011) Spatial and temporal correlation between neuron loss and neuroinflammation in a mouse model of neuronopathic Gaucher disease. Hum Mol Genet 20:1375–1386
Fillebeen C, Descamps L, Dehouck MP et al (1999) Receptor-mediated transcytosis of lactoferrin through the blood–brain barrier. J Biol Chem 274:7011–7017
Franke H, Galla HJ, Beuckmann CT (1999) An improved low-permeability in vitro-model of the blood–brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res 818:65–71
Fu H, Dirosario J, Killedar S, Zaraspe K, McCarty DM (2011) Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood–brain barrier gene delivery. Mol Ther 19:1025–1033
Garbuzova-Davis S, Louis MK, Haller EM, Derasari HM, Rawls AE, Sanberg PR (2011) Blood–brain barrier impairment in an animal model of MPS III B. PLoS One 6:e16601
Garcia MA, Carrasco M, Godoy A et al (2001) Elevated expression of glucose transporter-1 in hypothalamic ependymal cells not involved in the formation of the brain-cerebrospinal fluid barrier. J Cell Biochem 80:491–503
Ghosh C, Puvenna V, Gonzalez-Martinez J, Janigro D, Marchi N (2011) Blood–brain barrier P450 enzymes and multidrug transporters in drug resistance: a synergistic role in neurological diseases. Curr Drug Metab 12:742–749
Gleeson MP (2008) Generation of a set of simple, interpretable ADMET rules of thumb. J Med Chem 51:817–834
Grubb JH, Vogler C, Levy B, Galvin N, Tan Y, Sly WS (2008) Chemically modified beta-glucuronidase crosses blood–brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci U S A 105:2616–2621
Grubb JH, Vogler C, Sly WS (2010) New strategies for enzyme replacement therapy for lysosomal storage diseases. Rejuvenation Res 13:229–236
Guffon N, Bin-Dorel S, Decullier E, Paillet C, Guitton J, Fouilhoux A (2011) Evaluation of miglustat treatment in patients with type III mucopolysaccharidosis: a randomized, double-blind, placebo-controlled study. J Pediatr 159:838–844
Hamilton NB, Attwell D, Hall CN (2011) Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics 2:pii5 doi:10.3389/fnene.2010.00005
Hammarlund-Udenaes M, Fridén M, Syvänen S, Gupta A (2008) On the rate and extent of drug delivery to the brain. Pharm Res 25:1737–1750
Haqqani AS, Stanimirovic DB (2011) Intercellular interactomics of human brain endothelial cells and th17 lymphocytes: a novel strategy for identifying therapeutic targets of CNS inflammation. Cardiovasc Psychiatry Neurol 2011:175364
Hemsley KM, Hopwood JJ (2010) Lessons learnt from animal models: pathophysiology of neuropathic lysosomal storage disorders. J Inherit Metab Dis 33:363–371
Hervé F, Ghinea N, Scherrmann JM (2008) CNS delivery via adsorptive transcytosis. AAPS J 10:455–472
Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376
Ishikawa T, Saito H, Hirano H, Inoue Y, Ikegami Y (2012) Human ABC transporter ABCG2 in cancer chemotherapy: drug molecular design to circumvent multidrug resistance. Meth Mol Biol 910:267–278
Jolly RD, Marshall NR, Perrott MR, Dittmer KE, Hemsley KM, Beard H (2011) Intracisternal enzyme replacement therapy in lysosomal storage diseases: routes of absorption into brain. Neuropathol Appl Neurobiol 37:414–422
Jones AR, Shusta EV (2007) Blood–brain barrier transport of therapeutics via receptor-mediation. Pharm Res 24:1759–1771
Kandel ER, Schwartz J, Jessel T (2000) Principles of neural science, 4th edn. McGraw Hill, New York
Katona RL, Sinkó I, Holló G et al (2008) A combined artificial chromosome-stem cell therapy method in a model experiment aimed at the treatment of Krabbe’s disease in the Twitcher mouse. Cell Mol Life Sci 65:3830–3838
Killedar S, Dirosario J, Divers E, Popovich PG, McCarty DM, Fu H (2010) Mucopolysaccharidosis IIIB, a lysosomal storage disease, triggers a pathogenic CNS autoimmune response. J Neuroinflammation 7:39. doi:10.1186/1742-2094-7-39
Kloska A, Narajczyk M, Jakóbkiewicz-Banecka J et al (2012) Synthetic genistein derivatives as modulators of glycosaminoglycan storage. J Transl Med 10:153. doi:10.1186/1479-5876-10-153
Koshiba S, An R, Saito H, Wakabayashi K, Tamura A, Ishikawa T (2008) Human ABC transporters ABCG2 (BCRP) and ABCG4. Xenobiotica 38:863–888
Kovács R, Papageorgiou I, Heinemann U (2011) Slice cultures as a model to study neurovascular coupling and blood brain barrier in vitro. Cardiovasc Psychiatry Neurol 2011:646958. doi:10.1155/2011/646958
Liddelow SA, Temple S, Møllgård K et al (2012) Molecular characterisation of transport mechanisms at the developing mouse blood-CSF interface: a transcriptome approach. PLoS One 7:e33554
Lippmann ES, Azarin SM, Kay JE et al (2012) Derivation of blood–brain barrier endothelial cells from human pluripotent stem cells. Nat Biotechnol 30:783–791
Liu M, Hou T, Feng Z, Li Y (2012) The flexibility of P-glycoprotein for its poly-specific drug binding from molecular dynamics simulations. J Biomol Struct Dyn Aug 13. [Epub ahead of print] PubMed PMID: 22888853
Lockman PR, Mittapalli RK, Taskar KS et al (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16:5664–5678
Lohmann C, Hüwel S, Galla HJ (2002) Predicting blood–brain barrier permeability of drugs: evaluation of different in vitro assays. J Drug Target 10:263–276
Lundquist S, Renftel M, Brillault J, Fenart L, Cecchelli R, Dehouck MP (2002) Prediction of drug transport through the blood–brain barrier in vivo: a comparison between two in vitro cell models. Pharm Res 19:976–981
Malinowska M, Wilkinson FL, Langford-Smith KJ et al (2010) Genistein improves neuropathology and corrects behaviour in a mouse model of neurodegenerative metabolic disease. PLoS One 5:e14192
Markoutsa E, Pampalakis G, Niarakis A et al (2011) Uptake and permeability studies of BBB-targeting immunoliposomes using the hCMEC/D3 cell line. Eur J Pharm Biopharm 77:265–274
Markoutsa E, Papadia K, Clemente C, Flores O, Antimisiaris SG (2012) Anti-Aβ-MAb and dually decorated nanoliposomes: effect of Aβ1-42 peptides on interaction with hCMEC/D3 cells. Eur J Pharm Biopharm 81:49–56
Martin I (2004) Prediction of blood–brain barrier penetration: are we missing the point? Drug Discov Today 9:161–162
Matthes F, Wölte P, Böckenhoff A et al (2011) Transport of arylsulfatase A across the blood-brain barrier in vitro. J Biol Chem 286:17487–17494
Matzner U, Herbst E, Hedayati KK et al (2005) Enzyme replacement improves nervous system pathology and function in a mouse model for metachromatic leukodystrophy. Hum Mol Genet 14:1139–1152
Matzner U, Lüllmann-Rauch R, Stroobants S et al (2009) Enzyme replacement improves ataxic gait and central nervous system histopathology in a mouse model of metachromatic leukodystrophy. Mol Ther 17:600–606
Mayor S, Pagano RE (2007) Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8:603–612
Miller DS (2010) Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 31:246–254
Muldoon LL, Alvarez JI, Begley DJ et al (2013) Immunologic privilege in the central nervous system and the blood–brain barrier. J Cereb Blood Flow Metab 33:13–21. doi:10.1038/jcbfm.2012.153
Naik P, Cucullo L (2012) In vitro blood–brain barrier models: current and perspective technologies. J Pharm Sci 101:1337–1354
Nakagawa S, Deli MA, Kawaguchi H et al (2009) A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int 54:253–263
Neuwelt EA (2004) Mechanisms of disease: the blood–brain barrier. Neurosurgery 54:131–140, discussion 141–142
Neuwelt EA, Bauer B, Fahlke C et al (2011) Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 12:169–182
Ni Z, Bikadi Z, Rosenberg MF, Mao Q (2010) Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr Drug Metab 11:603–617
Ni Z, Bikadi Z, Shuster DL, Zhao C, Rosenberg MF, Mao Q (2011) Identification of proline residues in or near the transmembrane helices of the human breast cancer resistance protein (BCRP/ABCG2) that are important for transport activity and substrate specificity. Biochemistry 50:8057–8066
Ogunshola OO (2011) In vitro modeling of the blood–brain barrier: simplicity versus complexity. Curr Pharm Des 17:2755–2761
Ohtsuki S, Terasaki T (2007) Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res 24:1745–1758
Pajouhesh H, Lenz GR (2005) Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2:541–553
Pardridge WM, Eisenberg J, Cefalu WT (1985) Absence of albumin receptor on brain capillaries in vivo or in vitro. Am J Physiol 249:E264–E267
Parenti G, Pignata C, Vajro P, Salerno M (2013) New strategies for the treatment of lysosomal storage diseases (review). Int J Mol Med 31:11–20
Patabendige A, Skinner RA, Abbott NJ (2012) Establishment of a simplified in vitro porcine blood–brain barrier model with high transendothelial electrical resistance. Brain Res Jul 10. [Epub ahead of print] PubMed PMID: 22789905
Platt FM, Jeyakumar M (2008) Substrate reduction therapy. Acta Paediatr Suppl 97:88–93
Prinz M, Priller J, Sisodia SS, Ransohoff RM (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14:1227–1235
Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12:623–635
Redzic Z (2011) Molecular biology of the blood–brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS 8:3. doi:10.1186/2045-8118-8-3
Reichel A (2009) Addressing central nervous system (CNS) penetration in drug discovery: basics and implications of the evolving new concept. Chem Biodivers 6:2030–2049
Reichel A, Begley DJ, Abbott NJ (2003) An overview of in vitro techniques for blood–brain barrier studies. Meth Mol Med 89:307–324
Reinhardt RR, Bondy CA (1994) Insulin-like growth factors cross the blood–brain barrier. Endocrinology 135:1753–1761
Roberts AL, Fletcher JM, Moore L, Byers S (2010) Trans-generational exposure to low levels of rhodamine B does not adversely affect litter size or liver function in murine mucopolysaccharidosis type IIIA. Mol Genet Metab 101:208–213
Saha A, Sarkar C, Singh SP et al (2012) The blood–brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: amelioration by resveratrol. Hum Mol Genet 21:2233–2244
Saunders NR, Liddelow SA, Dziegielewska KM (2012) Barrier mechanisms in the developing brain. Front Pharmacol 3:46. doi:10.3389/fphar.2012.00046
Schiffmann R (2010) Therapeutic approaches for neuronopathic lysosomal storage disorders. J Inherit Metab Dis 33:373–379
Schultz ML, Tecedor L, Chang M, Davidson BL (2011) Clarifying lysosomal storage diseases. Trends Neurosci 34:401–410
Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV (2013) Deficiency in mural vascular cells coincides with blood–brain barrier disruption in Alzheimer’s disease. Brain Pathol 23:303–310
Shah KK, Yang L, Abbruscato TJ (2012) In vitro models of the blood–brain barrier. Meth Mol Biol 814:431–449
Shawahna R, Uchida Y, Declèves X et al (2011) and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm 8:1332–1341
Simionescu M, Popov D, Sima A (2009) Endothelial transcytosis in health and disease. Cell Tissue Res 335:27–40
Skinner RA, Gibson RM, Rothwell NJ, Pinteaux E, Penny JI (2009) Transport of interleukin-1 across cerebromicrovascular endothelial cells. Br J Pharmacol 156:1115–1123
Smith QR (2003) A review of blood–brain barrier transport techniques. Meth Mol Med 89:193–208
Spencer BJ, Verma IM (2007) Targeted delivery of proteins across the blood–brain barrier. Proc Natl Acad Sci U S A 104:7594–7599
Stamatovic SM, Sladojevic N, Keep RF, Andjelkovic AV (2012) Relocalization of junctional adhesion molecule A during inflammatory stimulation of brain endothelial cells. Mol Cell Biol 32:3414–3427
Stanimirovic DB, Friedman A (2012) Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab 32:1207–1221
Stroobants S, Gerlach D, Matthes F et al (2011) Intracerebroventricular enzyme infusion corrects central nervous system pathology and dysfunction in a mouse model of metachromatic leukodystrophy. Hum Mol Genet 20:2760–2769
Thanabalasundaram G, Schneidewind J, Pieper C, Galla HJ (2011) The impact of pericytes on the blood–brain barrier integrity depends critically on the pericyte differentiation stage. Int J Biochem Cell Biol 43:1284–1293
Tomanin R, Zanetti A, Zaccariotto E, D’Avanzo F, Bellettato CM, Scarpa M (2012) Gene therapy approaches for lysosomal storage disorders, a good model for the treatment of mendelian diseases. Acta Paediatr 101:692–701
Tosi G, Fano RA, Bondioli L et al (2011) Investigation on mechanisms of glycopeptide nanoparticles for drug delivery across the blood–brain barrier. Nanomedicine (Lond) 6:423–436
Tsinman O, Tsinman K, Sun N, Avdeef A (2011) Physicochemical selectivity of the BBB microenvironment governing passive diffusion-matching with a porcine brain lipid extract artificial membrane permeability model. Pharm Res 28:337–363
Tucker IG, Yang L, Mujoo H (2012) Delivery of drugs to the brain via the blood brain barrier using colloidal carriers. J Microencapsul 29:475–486
Urayama A, Grubb JH, Sly WS, Banks WA (2004) Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood–brain barrier. Proc Natl Acad Sci U S A 101:12658–12663
Vandenhaute E, Dehouck L, Boucau MC et al (2011) Modelling the neurovascular unit and the blood–brain barrier with the unique function of pericytes. Curr Neurovasc Res 8:258–269
Vercauteren D, Vandenbroucke RE, Jones AT et al (2010) The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther 18:561–569
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7:31–40
Visigalli I, Delai S, Politi LS et al (2010) Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood 116:5130–5139
Vitner EB, Platt FM, Futerman AH (2010) Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem 285:20423–20427
Vitner EB, Farfel-Becker T, Eilam R, Biton I, Futerman AH (2012) Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease. Brain 135:1724–1735
Vogler C, Levy B, Grubb JH et al (2005) Overcoming the blood–brain barrier with high-dose enzyme replacement therapy in murine mucopolysaccharidosis VII. Proc Natl Acad Sci U S A 102:14777–14782
Wang P, Xue Y, Shang X, Liu Y (2010) Diphtheria toxin mutant CRM197-mediated transcytosis across blood–brain barrier in vitro. Cell Mol Neurobiol 30:717–725
Wen CJ, Zhang LW, Al-Suwayeh SA, Yen TC, Fang JY (2012) Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. Int J Nanomedicine 7:1599–1611
Wilhelm I, Fazakas C, Krizbai IA (2011) In vitro models of the blood–brain barrier. Acta Neurobiol Exp (Wars) 71:113–128
Winkler EA, Bell RD, Zlokovic BV (2011) Central nervous system pericytes in health and disease. Nat Neurosci 14:1398–1405
Winkler EA, Sengillo JD, Bell RD, Wang J, Zlokovic BV (2012) Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cereb Blood Flow Metab 32:1841–1852
Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV (2013) Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 125:111–120
Wittkowski W (1998) Tanycytes and pituicytes: morphological and functional aspects of neuroglial interaction. Microsc Res Tech 41:29–42
Ylikangas H, Peura L, Malmioja K et al (2012) Structure-activity relationship study of compounds binding to large amino acid transporter 1 (LAT1) based on pharmacophore modeling and in situ rat brain perfusion. Eur J Pharm Sci 84:523–531. doi:10.1016/j.ejps.2012.11.014
Zlokovic BV (2005) Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 28:202–208
Zlokovic BV (2010) Neurodegeneration and the neurovascular unit. Nat Med 16:1370–1371
Acknowledgments
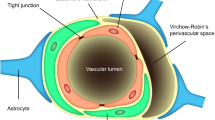
The author is grateful to Dr DJ Begley for discussion, and Dr SR Yusof for artwork on Fig. 3.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Maurizio Scarpa
Rights and permissions
About this article
Cite this article
Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36, 437–449 (2013). https://doi.org/10.1007/s10545-013-9608-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-013-9608-0