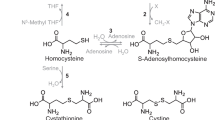
Abstract
Synthesis of cysteine as a product of the transsulfuration pathway can be viewed as part of methionine or homocysteine degradation, with cysteine being the vehicle for sulfur conversion to end products (sulfate, taurine) that can be excreted in the urine. Transsulfuration is regulated by stimulation of cystathionine β-synthase and inhibition of methylene tetrahydrofolate reductase in response to changes in the level of S-adenosylmethionine, and this promotes homocysteine degradation when methionine availability is high. Cysteine is catabolized by several desulfuration reactions that release sulfur in a reduced oxidation state, generating sulfane sulfur or hydrogen sulfide (H2S), which can be further oxidized to sulfate. Cysteine desulfuration is accomplished by alternate reactions catalyzed by cystathionine β-synthase and cystathionine γ-lyase. Cysteine is also catabolized by pathways that require the initial oxidation of the cysteine thiol by cysteine dioxygenase to form cysteinesulfinate. The oxidative pathway leads to production of taurine and sulfate in a ratio of approximately 2:1. Relative metabolism of cysteine by desulfuration versus oxidative pathways is influenced by cysteine dioxygenase activity, which is low in animals fed low-protein diets and high in animals fed excess sulfur amino acids. Thus, desulfuration reactions dominate when cysteine is deficient, whereas oxidative catabolism dominates when cysteine is in excess. In rats consuming a diet with an adequate level of sulfur amino acids, about two thirds of cysteine catabolism occurs by oxidative pathways and one third by desulfuration pathways. Cysteine dioxygenase is robustly regulated in response to cysteine availability and may function to provide a pathway to siphon cysteine to less toxic metabolites than those produced by cysteine desulfuration reactions.







Similar content being viewed by others
Abbreviations
- Met:
-
L-methionine
- Cys:
-
L-cysteine
- Hcy:
-
L-homocysteine
- SAA:
-
Sulfur-containing amino acids
- CBS:
-
Cystathionine β-synthase
- CSE:
-
Cystathionine γ-lyase
- CDO:
-
Cysteine dioxygenase
- CSD:
-
Cysteinesulfinate decarboxylase
References
Akagi R (1982) Purification and characterization of cysteine aminotransferase from rat liver cytosol. Acta Med Okayama 36:187–197
Bagley PJ, Stipanuk MH (1995) Rats fed a low protein diet supplemented with sulfur amino acids have increased cysteine dioxygenase activity and increased taurine production in hepatocytes. J Nutr 125:933–940
Banerjee R, Zou CG (2005) Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys 433:144–156
Bella DL, Stipanuk MH (1995) Effects of protein, methionine, or chloride on acid-base balance and on cysteine catabolism. Am J Physiol 269:E910–E917
Bella DL, Kwon YH, Stipanuk MH (1996) Variations in dietary protein but not in dietary fat plus cellulose or carbohydrate levels affect cysteine metabolism in rat isolated hepatocytes. J Nutr 126:2179–2187
Bella DL, Hahn C, Stipanuk MH (1999a) Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol 277:E144–E153
Bella DL, Hirschberger LL, Hosokawa Y, Stipanuk MH (1999b) Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am J Physiol 276:E326–E335
Bradley H, Gough A, Sokhi RS, Hassell A, Waring R, Emery P (1994) Sulfate metabolism is abnormal in patients with rheumatoid arthritis. Confirmation by in vivo biochemical findings. J Rheumatol 21:1192–1196
Brand A, Leibfritz D, Hamprecht B, Dringen R (1998) Metabolism of cysteine in astroglial cells: synthesis of hypotaurine and taurine. J Neurochem 71:827–832
Braunstein AE, Goryachenkova EV, Tolosa EA, Willhardt IH, Yefremova LL (1971) Specificity and some other properties of liver serine sulphhydrase: evidence for its identity with cystathionine β-synthase. Biochim Biophys Acta 242:247–260
Burlina A, Zacchello F, Dionisi-Vici C, Bertini E, Sabetta G, Bennet MJ, Hale DE, Schmidt-Sommerfeld E, Rinaldo P (1991) New clinical phenotype of branched-chain acyl-CoA oxidation defect. Lancet 338:1522–1523
Cavallini D, Mondovi B, De Marco C, Scioscia-Santoro A (1962a) Inhibitory effect of mercaptoethanol and hypotaurine on the desulfhydration of cysteine by cystathionase. Arch Biochem Biophys 96:456–457
Cavallini D, Mondovi B, De Marco C, Scioscia-Santoro A (1962b) The mechanism of desulphhydration of cysteine. Enzymologia 24:253–266
Chen X, Jhee KH, Kruger WD (2004) Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279:52082–52086
Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R (2009) H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284:11601–11612
Coloso RM, Stipanuk MH (1989) Metabolism of cyst(e)ine in rat enterocytes. J Nutr 119:1914–1924
Coloso RM, Drake MR, Stipanuk MH (1990) Effect of bathocuproine disulfonate, a copper chelator, on cyst(e)ine metabolism by freshly isolated rat hepatocytes. Am J Physiol 259:E443–E450
Crawhall JC (1985) A review of the clinical presentation and laboratory findings in two uncommon hereditary disorders ofsulfur amino acid metabolism, β-mercaptolactate cysteine disulfideuria and sulfite oxidase deficiency. Clin Biochem 18:139–142
Crawhall JC, Parker R, Sneddon W, Young EP (1969) Beta-mercaptolactate-cysteine disulfide in the urine of a mentally retarded patient. Am J Dis Child 117:71–82
Cresenzi CL, Lee JI, Stipanuk MH (2003) Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr 133:2697–2702
Davies MH, Ngong JM, Pean A, Vickers CR, Waring RH, Elias E (1995) Sulphoxidation and sulphation capacity in patients with primary biliary cirrhosis. J Hepatol 22:551–560
Dominy JE, Stipanuk MH (2004) New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr Rev 62:348–353
Dominy JE Jr, Hirschberger LL, Coloso RM, Stipanuk MH (2006a) Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26S proteasome system in the living rat. Biochem J 394:267–273
Dominy JE Jr, Hirschberger LL, Coloso RM, Stipanuk MH (2006b) In vivo regulation of cysteine dioxygenase via the ubiquitin-26S proteasome system. Adv Exp Med Biol 583:37–47
Dominy JE Jr, Simmons CR, Karplus PA, Gehring AM, Stipanuk MH (2006c) Identification and characterization of bacterial cysteine dioxygenases: a new route of cysteine degradation for eubacteria. J Bacteriol 188:5561–5569
Dominy JE Jr, Hwang J, Stipanuk MH (2007) Overexpression of cysteine dioxygenase reduces intracellular cysteine and glutathione pools in HepG2/C3A cells. Am J Physiol Endocrinol Metab 293:E62–E69
Dominy JE Jr, Hwang J, Guo S, Hirschberger LL, Zhang S, Stipanuk MH (2008) Synthesis of amino acid cofactor in cysteine dioxygenase is regulated by substrate and represents a novel post-translational regulation of activity. J Biol Chem 283:12188–12201
Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF (2002) Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicol Sci 65:18–25
Drake MR, De La Rosa J, Stipanuk MH (1987) Metabolism of cysteine in rat hepatocytes. Evidence for cysteinesulphinate-independent pathways. Biochem J 244:279–286
Ensunsa JL, Hirschberger LL, Stipanuk MH (1993) Catabolism of cysteine, cystine, cysteinesulfinate, and OTC by isolated perfused rat hindquarter. Am J Physiol 264:E782–789
Feng C, Tollin G, Enemark JH (2007) Sulfite oxidizing enzymes. Biochim Biophys Acta 1774:527–539
Fiedler H, Wood JL (1956) Specificity studies on the β-mercaptopyruvate-cyanide transsulfuration system. J Biol Chem 222:387–397
Finkelstein JD, Kyle WE, Harris BJ, Martin JJ (1982) Methionine metabolism in mammals: concentration of metabolites in rat tissues. J Nutr 112:1011–1018
Fiorucci S, Distrutti E, Cirino G, Wallace JL (2006) The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology 131:259–271
Garcia RA, Stipanuk MH (1992) The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr 122:1693–1701
García-Silva MT, Campos Y, Ribes A, Briones P, Cabello A, Santos Borbujo J, Arenas J, Garavaglia B (1994) Encephalopathy, petechiae, and acrocyanosis with ethylmalonic aciduria associated with muscle cytochrome c oxidase deficiency. J Pediatr 125:843–844
Goubern M, Andriamihaja M, Nübel T, Blachier F, Bouillaud F (2007) Sulfide, the first inorganic substrate for human cells. FASEB J 21:1699–1706
Guion-Rain MC, Portemer C, Chaatagner F (1975) Rat liver cysteine sulfinate decarboxylase: purification, new appraisal of the molecular weight and determination of catalytic properties. Biochim Biophys Acta 384:265–276
Hannestad U, Mårtensson J, Sjödahl R, Sörbo B (1981) 3-mercaptolactate cysteine disulfiduria: biochemical studies on affected and unaffected members of a family. Biochem Med 26:106–114
Heafield MT, Schmid D, Breitkreutz R, Stahl-Henning C, Drings P et al (1990) Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson’s and Alzheimer’s disease. Neurosci Lett 110:216–220
Hildebrandt TM, Grieshaber MK (2008) Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275:3352–3361
Huang J, Khan S, O’Brien PJ (1998) The glutathione dependence of inorganic sulfate formation from L- or D-cysteine in isolated rat hepatocytes. Chem Biol Interact 110:189–202
Ip MP, Thibert RJ, Schmidt DE Jr (1977) Purification and partial characterization of cysteine-glutamate transaminase from rat liver. Can J Biochem 55:958–964
Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH (2001) Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3:193–197
Janosík M, Kery V, Gaustadnes M, Maclean KN, Kraus JP (2001) Regulation of human cystathionine β-synthase by S-adenosyl-L-methionine: evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry 40:10625–10633
Johnson JL, Rajagopalan KV (1995) An HPLC assay for detection of elevated urinary S-sulphocysteine, a metabolic marker of sulphite oxidase deficiency. J Inherit Metab Dis 18:40–47
Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM Jr, Kirlin WG (2004) Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 18:1246–1248
Karlsen RL, Grofova I, Malthe-Sorenssen D, Fonnum F (1981) Morphological changes in rat brain induced by L-cysteine injection in newborn animals. Brain Res 208:167–180
Koj A, Frendo J, Janik Z (1967) [35S]Thiosulphate oxidation by rat liver mitochondria in the presence of glutathione. Biochem J 103:791–795
Kun E, Fanshier DW (1958) Isolation and identification of β-mercaptopyruvate desulfurase. Biochim Biophys Acta 27:659
Kun E, Fanshier DW (1959a) Enzymic transfer of sulfur from β-mercaptopyruvate to cyanide. Biochim Biophys Acta 33:26–18
Kun E, Fanshier DW (1959b) Isolation and properties of a β-mercaptopyruvate-cleaving copper enzyme. Biochim Biophys Acta 32:338–348
Kwon YH, Stipanuk MH (2001) Cysteine regulates expression of cysteine dioxygenase and γ-glutamylcysteine synthetase in cultured rat hepatocytes. Am J Physiol Endocrinol Metab 280:E804–815
Lak ND, Goryachenkova EV, Braunstein AE (1970) Investigation of substrate specificity of serine sulfhydrase from the hen liver and its relation to some inhibitors. Biokhimia 35:270–277
Lee JI, Londono M, Hirschberger LL, Stipanuk MH (2004) Regulation of cysteine dioxygenase and gamma-glutamylcysteine synthetase is associated with hepatic cysteine level. J Nutr Biochem 15:112–122
Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK, Levitt MD, Farrugia G, Szurszewski JH (2008) Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem 106:1577–15785
Lloyd D (2006) Hydrogen sulfide: clandestine microbial messenger? Trends Microbiol 14:456–462
Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P (2009) Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta 1787:864–872
Mathisen GA, Fonnum F, Paulsen RE (1996) Contributing mechanisms for cysteine excitotoxicity in cultured cerebellar granule cells. Neurochem Res 21:293–298
McCoy JG, Bailey LJ, Bitto E, Bingman CA, Aceti DJ, Fox BG, Phillips GN Jr (2006) Structure and mechanism of mouse cysteine dioxygenase. Proc Natl Acad Sci U S 103:3084–3089
Meister A, Fraser PE, Tice SV (1954) Enzymatic desulfuration of β-mercaptopyruvate to pyruvate. J Biol Chem 206:561–575
Mudd SH, Ebert MH, Scriver CR (1980) Labile methyl group balances in the human: the role of sarcosine. Metabolism 29:707–720
Mustafa AK, Gadalla MM, Snyder SH, Mustafa AK, Gadalla MM, Snyder SH (2009) Signaling by gasotransmitters. Sci Signal 2:re2
Niederwiesler A, Giliberti P, Baerlocher K (1973) β-Mercaptolactate cysteine disulfiduria in two normal sisters. Isolation and characterization of β-mercaptolactate cysteine disulfide. Clin Chim Acta 43:405–416
Oertel WH, Schmechel DE, Weise VK, Ransom DH, Tappax ML et al (1981) Comparison of cysteine sulphininc acid decarboxylase isoenzymes and glutamic acid decarboxylase in rat liver and brain. Neuroscience 6:2701–2714
Pedersen OO, Karlsen RL (1980) The toxic effect of L-cysteine on the rat retina. A morphological and biochemical study. Invest Ophthalmol Vis Sci 19:886–892
Perry TL, Krieger C, Hansen S, Tabatabaei A (1991) Amyotrophic lateral sclerosis: fasting plasma levels of cysteine and inorganic sulfate are normal, as are brain contents of cysteine. Neurology 41:487–490
Poole JR, Mudd SH, Conerly EB, Edwards WA (1975) Homocystinuria due to cystathionine synthase deficiency: studies of nitrogen balance and sulfur excretion. J Clin Invest 55:1033–1048
Porter PN, Grishaver MS, Jones OW (1974) Characterization of human cystathionine β-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine β-synthase. Biochim Biophys Acta 364:128–139
Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R (2006) S-adenosylmethionine stabilizes cystathionine β-synthase and modulates redox capacity. Proc Natl Acad Sci U S A 103:6489–6494
Qu K, Lee SW, Bian JS, Low CM, Wong PT (2008) Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 52:155–165
Recasens M, Benezra R, Basset P, Mandel P (1980) Cysteine sulfinate aminotransferase and aspartate aminotransferase isoenzymes of rat brain: purification, characterization, and further evidence for identity. Biochemistry 19:4583–4589
Rentschler LA, Hirschberger LL, Stipanuk MH (1986) Response of the kitten to dietary taurine depletion: effects on renal reabsorption, bile acid conjugation and activities of enzymes involved in taurine synthesis. Comp Biochem Physiol B 84:319–325
Rupar CA, Gillettt J, Gordon BA, Ramsay DA, Johnson JL et al (1996) Isolated sulfite oxidase deficiency. Neuropediatrics 27:299–304
Sandberg M, Orwar O, Hehmann A (1991) L-Cysteine toxicity: effects on non-sulfur amino acids, sulfur-containing excitatory amino acids and γ-glutamyl peptides in the immature brain. J Neurochem 57:S152
Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11:703–714
Simmons CR, Hirschberger LL, Machi MS, Stipanuk MH (2006a) Expression, purification, and kinetic characterization of recombinant rat cysteine dioxygenase, a non-heme metalloenzyme necessary for regulation of cellular cysteine levels. Protein Expr Purif 47:74–81
Simmons CR, Liu Q, Huang Q, Hao Q, Begley TP, Karplus PA, Stipanuk MH (2006b) Crystal structure of mammalian cysteine dioxygenase. A novel mononuclear ironcenter for cysteine thiol oxidation. J Biol Chem 281:18723–18733
Simmons CR, Krishnamoorthy K, Granett SL, Schuller DJ, Dominy JE Jr, Begley TP, Stipanuk MH, Karplus PA (2008) A putative Fe2+-bound persulfenate intermediate in cysteine dioxygenase. Biochemistry 47:11390–11392
Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R (2009) The relative contributions of cystathionine β-synthase and γ -cystathionase to H2S biogenesis via alternative transsulfuration reactions. J Biol Chem 284:22457–22466
Sörbo B (1987) 3-Mercaptopyruvate, 3-mercaptolactate and mercaptoacetate. Methods Enzymol 143:178–182
Stipanuk MH (1986) Metabolism of sulfur-containing amino acids. Annu Rev Nutr 6:179–209
Stipanuk MH (2004) Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:539–577
Stipanuk MH, Beck PW (1982) Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206:267–277
Stipanuk MH, Rotter MA (1984) Metabolism of cysteine, cysteinesulfinate and cysteinesulfonate in rats fed adequate and excess levels of sulfur-containing amino acids. J Nutr 114:1426–1437
Stipanuk MH, De la Rosa J, Hirschberger LL (1990) Catabolism of cyst(e)ine by rat renal cortical tubules. J Nutr 120:450–458
Stipanuk MH, Londono M, Lee JI, Hu M, Yu AF (2002) Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr 132:3369–3378
Stipanuk MH, Hirschberger LL, Londono MP, Cresenzi CL, Yu AF (2004a) The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am J Physiol Endocrinol Metab 286:E439–E448
Stipanuk MH, Londono M, Hirschberger LL, Hickey C, Thiel DJ, Wang L (2004b) Evidence for expression of a single distinct form of mammalian cysteine dioxygenase. Amino Acids 26:99–106
Stipanuk MH, Dominy JE Jr, Lee JI, Coloso RM (2006) Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136:1652S–1659S
Storch KJ, Wagner DA, Burke JF, Young VR (1988) Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1–13C]methionine. Am J Physiol 255:E322–E331
Szczepkowski TW, Wood JL (1967) The cystathionase-rhodanese system. Biochim Biophys Acta 139:469–478
Szczepkowski TW, Skarzynski B, Weber M (1961) The metabolic state of thiosulphate. Nature 189:1007–1008
Taniguchi T, Kimura T (1974) Role of 3-mercaptopyruvate sulfurtransferase in the formation of the iron-sulfur chromophore of adrenal ferredoxin. Biochim Biophys Acta 364:284–295
Tiranti V, Viscomi C, Hildebrandt T, Di Meo I, Mineri R, Tiveron C, Levitt MD, Prelle A, Fagiolari G, Rimoldi M, Zeviani M (2009) Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med 15:200–205
Touati G, Rusthoven E, Depondt E, Dorche C, Duran M, Heron B, Rabier D, Russo M, Saudubray JM (2000) Dietary therapy in two patients with a mild form of sulphite oxidase deficiency. Evidence for clinical and biological improvement. J Inherit Metab Dis 23:45–53
Ubuka T, Umemura S, Ishimoto Y, Shimomura M (1977a) Transaminase of L-cysteine in rat liver mitochondria. Physiol Chem Phys 9:91–96
Ubuka T, Yuasa S, Ishimoto Y, Shimomura M (1977b) Desulfuration of L-cysteine through transamination and transsulfuration in rat liver. Physiol Chem Phys 9:241–246
Ubuka T, Umemura S, Yuasa S, Kinuta M, Watanabe K (1978) Purification and characterization of mitochondrial cysteine aminotransferase from rat liver. Physiol Chem Phys 10:483–500
Ueki I, Stipanuk MH (2009) 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J Nutr 139:207–214
Uren JR, Ragin R, Chaykovsky M (1978) Modulation of cysteine metabolism in mice-effects of propargylglycine and L-cyst(e)ine-degrading enzymes. Biochem Pharmacol 27:2807–2814
Wang R (2002) Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Wang XB, Jin HF, Tang CS, Du JB (2009) Significance of endogenous sulfur-containing gases in the cardiovascular system. Clin Exp Pharmacol Physiol.. doi:10.1111/j.1440-1681.2009.05249
Yagi T, Kagamiyama H, Nozaki M (1979) Cysteine sulfinate transamination activity of aspartate aminotransferase. Biochem Biophys Res Commun 90:447–452
Yamaguchi K, Hosokawa Y, Kohashi N, Kori Y, Sakakibara S, Ueda I (1978) Rat liver cysteine dioxygenase (cysteine oxidase). Further purification, characterization, and analysis of the activation and inactivation. J Biochem 83:479–491
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R (2008) H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322:587–590
Yao K, Kinuta M, Akagi R (1979) Cat liver cystathionase. Physiol Chem Phys 11:257–260
Ye S, Wu X, Wei L, Tang D, Sun P, Bartlam M, Rao Z (2007) An insight into the mechanism of human cysteine dioxygenase. Key roles of the thioether-bonded tyrosine-cysteine cofactor. J Biol Chem 282:3391–3402
Acknowledgement
This work was supported in part by grant DK056649 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated editor: Viktor Kozich
Competing interest: none declared.
Rights and permissions
About this article
Cite this article
Stipanuk, M.H., Ueki, I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis 34, 17–32 (2011). https://doi.org/10.1007/s10545-009-9006-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-009-9006-9