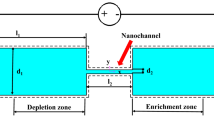
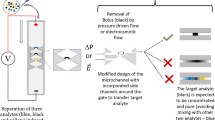
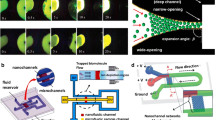
Abstract
This review reports the progress on the recent development of electrokinetic enrichment in micro-nanofluidic chips. The governing equations of electrokinetic enrichment in micro-nanofluidic chips are given. Various enrichment applications including protein analysis, DNA analysis, bacteria analysis, viruses analysis and cell analysis are illustrated and discussed. The advantages and difficulties of each enrichment method are expatiated. This paper will provide a particularly convenient and valuable reference to those who intend to research the electrokinetic enrichment based on micro-nanofluidic chips.




Similar content being viewed by others
References
K. Aïzel, V. Agache, C. Pudda, et al., Enrichment of nanoparticles and bacteria using electroless and manual actuation modes of a bypass nanofluidic device[J]. Lab Chip 13(22), 4476–4485 (2013)
M. Arca, A.J.C. Ladd, J.E. Butler, Electro-hydrodynamic concentration of genomic length DNA[J]. Soft Matter 12(33), 6975–6984 (2016)
P. Augustsson, C. Magnusson, M. Nordin, et al., Microfluidic, label-free enrichment of prostate cancer cells in blood based on acoustophoresis[J]. Anal. Chem. 84(18), 7954–7962 (2012)
G. Bruno, G. Canavese, X. Liu, et al., The active modulation of drug release by an ionic field effect transistor for an ultra-low power implantable nanofluidic system[J]. Nano 8(44), 18718–18725 (2016)
X.Y. Chen, T.C. Li, A novel passive micromixer designed by applying an optimization algorithm to the zigzag microchannel[J]. Chem. Eng. J. (2016). doi:10.1016/j.cej.2016.11.052
G.D. Chen, C.J. Alberts, W. Rodriguez, et al., Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles[J]. Anal. Chem. 82(2), 723–728 (2009)
Y.W. Chen, H. Wang, M. Hupert, et al., Modular microfluidic system fabricated in thermoplastics for the strain-specific detection of bacterial pathogens[J]. Lab Chip 12(18), 3348–3355 (2012)
Y.Y. Chen, P.H. Chiu, C.H. Weng, et al., Preconcentration of diluted mixed-species samples following separation and collection in a micro–nanofluidic device[J]. Biomicrofluidics 10(1), 014119 (2016)
J. Dai, T. Ito, L. Sun, et al., Electrokinetic trapping and concentration enrichment of DNA in a microfluidic channel[J]. J. Am. Chem. Soc. 125(43), 13026–13027 (2003)
H. Daiguji, P. Yang, A. Majumdar, Ion transport in nanofluidic channels[J]. Nano Lett. 4(1), 137–142 (2004)
T.F. Didar, M. Tabrizian, Adhesion based detection, sorting and enrichment of cells in microfluidic lab-on-Chip devices[J]. Lab Chip 10(22), 3043–3053 (2010)
J.R. Du, H.H. Wei, Focusing and trapping of DNA molecules by head-on ac electrokinetic streaming through join asymmetric polarization[J]. Biomicrofluidics 4(3), 034108 (2010)
M. Elitas, R. Martinez-Duarte, N. Dhar, et al., Dielectrophoresis-based purification of antibiotic-treated bacterial subpopulations[J]. Lab Chip 14(11), 1850–1857 (2014)
J.V. Green, S.K. Murthy, Microfluidic enrichment of a target cell type from a heterogenous suspension by adhesion-based negative selection[J]. Lab Chip 9(15), 2245–2248 (2009)
J. Grimme, T. King, K.D. Jo, et al., Development of Fieldable lab-on-a-Chip Systems for detection of a broad Array of targets from toxicants to Biowarfare agents[J]. J. Nanotechnol Eng Med 4(2), 020904 (2013)
S.C. Hur, N.K. Henderson-MacLennan, E.R.B. McCabe, et al., Deformability-based cell classification and enrichment using inertial microfluidics[J]. Lab Chip 11(5), 912–920 (2011a)
S.C. Hur, A.J. Mach, D. Di Carlo, High-throughput size-based rare cell enrichment using microscale vortices[J]. Biomicrofluidics 5(2), 022206 (2011b)
C.P. Jen, T.G. Amstislavskaya, K.F. Chen, et al., Sample preconcentration utilizing nanofractures generated by junction gap breakdown assisted by self-assembled monolayer of gold nanoparticles[J]. PLoS One 10(5), e0126641 (2015)
X. Jin, S. Joseph, E.N. Gatimu, et al., Induced electrokinetic transport in micro-nanofluidic interconnect devices[J]. Langmuir 23(26), 13209–13222 (2007)
W. Jing, W. Zhao, S. Liu, et al., Microfluidic device for efficient airborne bacteria capture and enrichment[J]. Anal. Chem. 85(10), 5255–5262 (2013)
S.M. Kim, M.A. Burns, E.F. Hasselbrink, Electrokinetic protein preconcentration using a simple glass/poly (dimethylsiloxane) microfluidic chip[J]. Anal. Chem. 78(14), 4779–4785 (2006)
S.J. Kim, Y.A. Song, J. Han, Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications[J]. Chem. Soc. Rev. 39(3), 912–922 (2010)
J. Kim, J.P. Hilton, K.A. Yang, et al., Nucleic acid isolation and enrichment on a microchip[J]. Sensors Actuators A Phys. 195, 183–190 (2013)
K.W. Kwon, S.S. Choi, S.H. Lee, et al., Label-free, microfluidic separation and enrichment of human breast cancer cells by adhesion difference[J]. Lab Chip 7(11), 1461–1468 (2007)
K.T. Liao, C.F. Chou, Nanoscale molecular traps and dams for ultrafast protein enrichment in high-conductivity buffers[J]. J. Am. Chem. Soc. 134(21), 8742–8745 (2012)
Masuda T, Maruyama H, Honda A, et al. Active virus filter for enrichment and manipulation of virus[C]//Micro Electro Mechanical Systems (MEMS), 2011 I.E. 24th international conference on. IEEE, 2011: 1099–1102
S.K. Murthy, P. Sethu, G. Vunjak-Novakovic, et al., Size-based microfluidic enrichment of neonatal rat cardiac cell populations[J]. Biomed. Microdevices 8(3), 231–237 (2006)
M. Napoli, J.C.T. Eijkel, S. Pennathur, Nanofluidic technology for biomolecule applications: A critical review[J]. Lab Chip 10(8), 957–985 (2010)
K.H. Paik, Y. Liu, V. Tabard-Cossa, et al., Control of DNA capture by nanofluidic transistors[J]. ACS Nano 6(8), 6767–6775 (2012)
M.C. Park, M. Kim, G.T. Lim, et al., Droplet-based magnetic bead immunoassay using microchannel-connected multiwell plates (μCHAMPs) for the detection of amyloid beta oligomers[J]. Lab Chip 16(12), 2245–2253 (2016)
C. Peskoller, R. Niessner, M. Seidel, Cross-flow microfiltration system for rapid enrichment of bacteria in water[J]. Anal. Bioanal. Chem. 393(1), 399–404 (2009)
J.A. Phillips, Y. Xu, Z. Xia, et al., Enrichment of cancer cells using aptamers immobilized on a microfluidic channel[J]. Anal. Chem. 81(3), 1033–1039 (2008)
S. Podszun, P. Vulto, H. Heinz, et al., Enrichment of viable bacteria in a micro-volume by free-flow electrophoresis[J]. Lab Chip 12(3), 451–457 (2012)
H. Ranchon, R. Malbec, V. Picot, et al., DNA separation and enrichment using electro-hydrodynamic bidirectional flows in viscoelastic liquids[J]. Lab Chip 16(7), 1243–1253 (2016)
A. Sin, S.K. Murthy, A. Revzin, et al., Enrichment using antibody-coated microfluidic chambers in shear flow: Model mixtures of human lymphocytes[J]. Biotechnol. Bioeng. 91(7), 816–826 (2005)
H. Song, Y. Wang, C. Garson, et al., Nafion-film-based micro–nanofluidic device for concurrent DNA preconcentration and separation in free solution[J]. Microfluid. Nanofluid. 17(4), 693–699 (2014)
D. Stein, Z. Deurvorst, F.H.J. van der Heyden, et al., Electrokinetic concentration of DNA polymers in nanofluidic channels[J]. Nano Lett. 10(3), 765–772 (2010)
K.B. Sung, K.P. Liao, Y.L. Liu, et al., Development of a nanofluidic preconcentrator with precise sample positioning and multi-channel preconcentration[J]. Microfluid. Nanofluid. 14(3–4), 645–655 (2013)
Y.C. Wang, J. Han, Pre-binding dynamic range and sensitivity enhancement for immuno-sensors using nanofluidic preconcentrator[J]. Lab Chip 8(3), 392–394 (2008)
Wang J, Liu C. Electrokinetic ion transport in confined micro-nanochannel[J]. Electrophoresis, 2016.
Y.C. Wang, A.L. Stevens, J. Han, Million-fold preconcentration of proteins and peptides by nanofluidic filter[J]. Anal. Chem. 77(14), 4293–4299 (2005)
C. Wang, J. Ouyang, H.L. Gao, et al., UV-ablation nanochannels in micro/nanofluidics devices for biochemical analysis[J]. Talanta 85(1), 298–303 (2011)
C. Wang, J. Ouyang, Y.Y. Wang, et al., Sensitive assay of protease activity on a micro/Nanofluidics Preconcentrator fused with the fluorescence resonance energy transfer detection technique[J]. Anal. Chem. 86(6), 3216–3221 (2014)
C. Wang, Y. Shi, J. Wang, et al., Ultrasensitive protein concentration detection on a micro/Nanofluidic enrichment Chip using fluorescence quenching[J]. ACS Appl. Mater. Interfaces 7(12), 6835–6841 (2015)
Z. Wu, B. Willing, J. Bjerketorp, et al., Soft inertial microfluidics for high throughput separation of bacteria from human blood cells[J]. Lab Chip 9(9), 1193–1199 (2009)
Z.Y. Wu, F. Fang, Y.Q. He, et al., Flexible and efficient eletrokinetic stacking of DNA and proteins at an HF etched porous junction on a fused silica capillary[J]. Anal. Chem. 84(16), 7085–7091 (2012)
Y. Xu, J.A. Phillips, J. Yan, et al., Aptamer-based microfluidic device for enrichment, sorting, and detection of multiple cancer cells[J]. Anal. Chem. 81(17), 7436–7442 (2009)
L. Yang, P.P. Banada, M.R. Chatni, et al., A multifunctional micro-fluidic system for dielectrophoretic concentration coupled with immuno-capture of low numbers of listeria monocytogenes[J]. Lab Chip 6(7), 896–905 (2006)
H. Yu, Y. Lu, Y. Zhou, et al., A simple, disposable microfluidic device for rapid protein concentration and purification via direct-printing[J]. Lab Chip 8(9), 1496–1501 (2008)
B. Zhu, J. Smith, M.L. Yarmush, et al., Microfluidic enrichment of mouse epidermal stem cells and validation of stem cell proliferation in vitro[J]. Tissue Eng Part C: Methods 19(10), 765–773 (2013)
Acknowledgements
This work was supported by Liaoning Province Doctor Startup Fund (20141131) and Fund of Liaoning Province Education Administration (L2014241).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Zhang, S., Zhang, L. et al. Applications and theory of electrokinetic enrichment in micro-nanofluidic chips. Biomed Microdevices 19, 19 (2017). https://doi.org/10.1007/s10544-017-0168-1
Published:
DOI: https://doi.org/10.1007/s10544-017-0168-1