Abstract
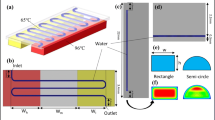
A novel continuous-flow polymerase chain reaction (PCR) chip has been analyzed in our work. Two temperature zones are controlled by two external controllers and the other temperature zone at the chip center is controlled by the flow rate of the fluid inside a channel under the glass chip. By employing a water cooling channel at the chip center, the sequence of denaturation, annealing, and extension can be created due to the forced convection effect. The required annealing temperature of PCR less than 313 K can also be demonstrated in this chip. The Poly(methyl methacrylate) (PMMA) cooling channel with the thin aluminum cover is utilized to enhance the temperature uniformity. The size of this chip is 76 mm × 26 mm × 3 mm. This device represents the first demonstration of water cooling thermocycling within continuous-flow PCR microfluidics. The commercial software CFD-ACE+TM is utilized to determine the distances between the heating assemblies within the chip. We investigate the influences of various chip materials, operational parameters of the cooling channel and geometric parameters of the chip on the temperature uniformity on the chip surface. Concerning the temperature uniformity of the working zones and the lowest temperature at the annealing zone, the air gap spacing of 1 mm and the cooling channel thicknesses of 1 mm of the PMMA channel with an aluminum cover are recommended in our design. The hydrophobic surface of the PDMS channel was modified by filling it with 20 % Tween 20 solution and then adding bovine serum albumin (BSA) solution to the PCR mixture. DNA fragments with different lengths (372 bp and 478 bp) are successfully amplified with the device.
















Similar content being viewed by others
References
J.J. Chen, Y.T. Yang, Modeling and experiment of shuttling speed effects on the OSTRYCH. Appl. Therm. Eng. 31, 2797–2807 (2011)
P.C. Chen, D.E. Nikitopoulos, S.A. Soper, M.C. Murphy, Temperature distribution effects on micro-CFPCR performance. Biomed. Microdevices 10, 141–152 (2008)
K.H. Chung, S.H. Park, Y.H. Choi, A palmtop PCR system with a disposable polymer chip operated by the thermosiphon effect. Lab Chip 10, 202–210 (2010)
T.H. Fang, N. Ramalingam, D. Xian-Dui, T.S. Ngin, Z. Xianting, A.T. Lai Kuan, E.Y. Peng Huat, G. Hai-Qing, Real-time PCR microfluidic devices with concurrent electrochemical detection. Biosens. Bioelectron. 24, 2131–2136 (2009)
J. Felbel, A. Reichert, M. Kielpinski, M. Urban, T. Henkel, N. Häfner, M. Dürst, J. Weber, Reverse transcription-polymerase chain reaction (RT-PCR) in flow-through micro-reactors: thermal and fluidic concepts. Chem. Eng. J. 135S, S298–S302 (2008)
Y. Fuchiwaki, H. Nagai, M. Saito, E. Tamiya, Ultra-rapid flow-through polymerase chain reaction microfluidics using vapor pressure. Biosens. Bioelectron. 27, 88–94 (2011)
M. Hashimoto, P.C. Chen, M.W. Mitchell, D.E. Nikitopoulos, S.A. Soper, M.C. Murphy, Rapid PCR in a continuous flow device. Lab Chip 4, 638–645 (2004)
J.P. Holman, Heat Transfer, 10th edn. (McGraw-Hill, New York, 2009)
T.M. Hsieh, C.H. Luo, F.C. Huang, J.H. Wang, L.J. Chien, G.B. Lee, Enhancement of thermal uniformity for a microthermal cycler and its application for polymerase chain reaction. Sensors Actuators B Chem. 130, 848–856 (2008)
J.A. Kim, J.Y. Lee, S. Seong, S.H. Cha, S.H. Lee, J.J. Kim, T.H. Park, Fabrication and characterization of a PDMS-glass hybrid continuous-flow PCR chip. Biochem. Eng. J. 29, 91–97 (2006)
M.U. Kopp, A.J.D. Mello, A. Manz, Chemical amplification: continuous-flow PCR on a chip. Science 280, 1046–1048 (1998)
Y. Li, C. Zhang, D. Xing, Fast identification of foodborne pathogenic viruses using continuous-flow reverse transcription-PCR with fluorescence detection. Microfluid. Nanofluid. 10, 367–380 (2011)
D.P. Manage, Y.C. Morrissey, A.J. Stickel, J. Lauzon, A. Atrazhev, J.P. Acker, L.M. Pilarski, On-chip PCR amplification of genomic and viral templates in unprocessed whole blood. Microfluid. Nanofluid. 10, 697–702 (2011)
S. Mohr, Y.H. Zhang, A. Macaskill, P.J.R. Day, R.W. Barber, N.J. Goddard, D.R. Emerson, P.R. Fielden, Numerical and experimental study of a droplet-based PCR chip. Microfluid. Nanofluid. 3, 611–621 (2007)
T. Nakayama, H.M. Hiep, S. Furui, Y. Yonezawa, M. Saito, Y. Takamura, E. Tamiya, An optimal design method for preventing air bubbles in high-temperature microfluidic devices. Anal. Bioanal. Chem. 396, 457–464 (2010)
M.A. Northrup, M.T. Ching, R.M. White, R.T. Wltson (1993) DNA amplification in a microfabricated reaction chamber. In: Proceeding of the 7th international conference of solid state sensors and actuators, Yokohama, Japan, pp 924–926
P.J. Obeid, T.K. Christopoulos, Continuous-flow DNA and RNA amplification chip combined with laser-induced fluorescence detection. Anal. Chim. Acta 494, 1–9 (2003)
X. Qiu, M.G. Mauk, D. Chen, C. Liu, H.H. Bau, A large volume, portable, real-time PCR reactor. Lab Chip 10, 3170–3177 (2010)
R.K. Saiki, S. Scharf, F. Faloona, K.B. Mullis, G.T. Horn, H.A. Erlich, N. Arnheim, Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1950–1954 (1985)
I. Schneegaß, R. Bräutigam, J.M. Köhler, Miniaturized flow-through PCR with different template types in a silicon chip thermocycler. Lab Chip 1, 42–49 (2001)
C.Y. Shih, Y. Chen, Y.C. Tai, Parylene-strengthened thermal isolation technology for microfluidic system-on-chip applications. Sensors Actuators A Phys. 126, 270–276 (2006)
K. Sun, A. Yamaguchi, Y. Ishida, S. Matsuo, H. Misawa, A heater-integrated transparent microchannel chip for continuous-flow PCR. Sensors Actuators B Chem. 84, 283–289 (2002)
W. Wang, Z.X. Li, R. Luo, S.H. Lü, A.D. Xu, Y.J. Yang, Droplet-based micro oscillating-flow PCR chip. J. Micromech. Microeng. 15, 1369–1377 (2005)
C.T. Wittwer, D.J. Garling, Rapid cycle DNA amplification: time and temperature optimization. Biotechniques 10, 76–83 (1991)
M. Yang, R. Pal, M.A. Burns, Cost-effective thermal isolation techniques for use on microfabricated DNA amplification and analysis devices. J. Micromech. Microeng. 15, 221–230 (2005)
L. Yao, B. Liu, T. Chen, S. Liu, T. Zuo, Micro flow-through PCR in a PMMA chip fabricated by KrF excimer laser. Biomed. Microdevices 7, 253–257 (2005)
C. Yu, W. Liang, I. Kuan, C. Wei, W. Gu, Fabrication and characterization of a flow-through PCR device with integrated chromium resistive heaters. J. Chin. Inst. Chem. Eng. 38, 333–339 (2007)
C. Zhang, D. Xing, Microfluidic gradient PCR (MG-PCR): a new method for microfluidic DNA amplification. Biomed. Microdevices 12, 1–12 (2010)
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China for financially supporting this research under Contract No. NSC 100-2221-E-020-024-. Daryl Switak is appreciated for his editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J.J., Shen, C.M. & Ko, Y.W. Analytical study of a microfludic DNA amplification chip using water cooling effect. Biomed Microdevices 15, 261–278 (2013). https://doi.org/10.1007/s10544-012-9728-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-012-9728-6