Abstract
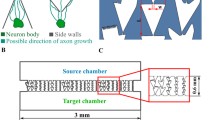
We present the design, analysis, construction, and culture results of a microfluidic device for the segregation and chemical stimulation of primary rat hippocampal neurons. Our device is designed to achieve spatio-temporal solute delivery to discrete sections of neurons with mitigated mechanical stress. We implement a geometric guidance technique to direct axonal processes of the neurons into specific areas of the device to achieve solute segregation along routed cells. Using physicochemical modeling, we predict flows, concentration profiles, and mechanical stresses within pertiment sections of the device. We demonstrate cell viability and growth within the closed device over a period of 11 days. Additionally, our modeling methodology may be generalized and applied to other device geometries.







Similar content being viewed by others
References
G. Banker, K. Goslin (eds.), Culturing nerve cells (MIT Press, 1998)
R. B. Campenot, Local Control of Neurite Development by Nerve Growth-Factor. P Nat. Acad. Sci. USA 74(10), 4516–4519 (1977)
R.B. Campenot, Development of sympathetic neurons in compartmentalized cultures 1. local-control of neurite growth by nerve growth-factor. Dev. Biol. 93(1), 1–12 (1982a)
R.B. Campenot, Development of sympathetic neurons in compartmentalized cultures 2. local-control of neurite survival by nerve growth-factor. Dev. Biol. 93(1), 13–21 (1982b)
K.J. De Vos, A.J. Grierson, S. Ackerley, C.C. Miller, Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31(1), 151–173 (2008)
P.G. Gross, E.P. Kartalov, A. Scherer, L.P. Weiner, Applications of microfluidics for neuronal studies. J. Neurol. Sci. 252(2), 135–143 (2007)
C. James, R. Davis, M. Meyer, A. Turner, S. Turner, G. Withers, L. Kam, G. Banker, H. Craighead, M. Issacson, J. Turner, W. Shain, Aligned microcontact printing of micrometer-scale poly-l-lysine structures for controlled growth of cultured neurons on planar microelectrode arrays. IEEE Trans. Biomed. Eng. 47(1), 17–21 (2000)
S. Kaech, G. Banker, Culturing hippocampal neurons. Nat. Protoc. 1(5), 2406–2415 (2006)
S. Kaech, C.F. Huang, G. Banker, Short-term high-resolution imaging of developing hippocampal neurons in culture. Cold Spring Harb. Protoc. (2012). doi:10.1101/pdb.prot068247
T.M. Keenan, A. Folch, Biomolecular gradients in cell culture systems. Lab Chip 8, 34–57 (2008)
B.J. Kirby, Micro- and Nanoscale Fluid Mechanics Transport in Microfluidic Devices (Cambridge University Press, 2011)
D. Kleinfeld, K. Kahler, P. Hockberger, Controlled outgrowth of dissociated neurons on patterned substrates. J. Neurosci. 8(11), 4098–4120 (1988)
A. Kunze, R. Meissner, S. Brando, P. Renaud, Co-pathological connected primary neurons in a microfluidic device for alzheimer studies. Biotechnol. Bioeng. 108(9), 2241–2245 (2011)
S.P. Lacour, R. Atta, J.J. FitzGerald, M. Blamire, E. Tarte, J. Fawcett, Polyimide micro-channel arrays for peripheral nerve regenerative implants. Sens. Actuators, A, Phys. 147(2), 456–463 (2008)
I. Meyvantsson, D.J. Beebe, Cell culture models in microfluidic systems. Annu. Rev. Anal. Chem. 1(1), 423–449 (2008)
L.J. Millet, M.E. Stewart, J.V. Sweedler, R.G. Nuzzo, M.U. Gillette, Microfluidic devices for culturing primary mammalian neurons at low densities. Lab Chip 7, 987–994 (2007)
J. Monahan, A.A. Gewirth, R.G. Nuzzo, A method for filling complex polymeric microfluidic devices and arrays. Anal. Chem. 73(13), 3193–3197 (2001)
M.P. Murphy, How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009)
A.A. Oliva, C.D. James, C.E. Kingman, H.G. Craighead, G.A. Banker, Patterning axonal guidance molecules using a novel strategy for microcontact printing. Neurochem. Res. 28, 1639–1648 (2003)
T.M. Pearce, J.C. Williams, Microtechnology: Meet neurobiology. Lab Chip 7, 30–40 (2007)
J.M. Peyrin, B. Deleglise, L. Saias, M. Vignes, P. Gougis, S. Magnifico, S. Betuing, M. Pietri, J. Caboche, P. Vanhoutte, J.L. Viovy, B. Brugg, Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab on a Chip 11(21), 3663–3673 (2011)
R.B. Schoch, J. Han, P. Renaud, Transport phenomena in nanofluidics. Rev. Mod. Phys. 80, 839–883 (2008)
R. Selvatici, M. Previati, S. Marino, L. Marani, S. Falzarano, I. Lanzoni, A. Siniscalchi, Sodium azide induced neuronal damage in vitro: evidence for non-apoptotic cell death. Neurochem. Res. 34, 909–916 (2009)
T.M. Squires, S.R. Quake, Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 77, 977–1026 (2005)
C. Szabo, H. Ischiropoulos, R. Radi, Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev., Drug Discov. 6(8), 662–680 (2007)
A.M. Taylor, M. Blurton-Jones, S.W. Rhee, D.H. Cribbs, C.W. Cotman, N.L. Jeon, A microfluidic culture platform for cns axonal injury, regeneration and transport. Nat. Methods 2(8), 599–605 (2005)
A.M. Taylor, S.W. Rhee, C.H. Tu, D.H. Cribbs, C.W. Cotman, N.L. Jeon, Microfluidic multicompartment device for neuroscience research. Langmuir 19(5), 1551–1556 (2003)
D. Wallace, A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005)
C.J. Wang, X. Li, B. Lin, S. Shim, G.l. Ming, A. Levchenko, A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound ecm molecules and diffusible guidance cues. Lab Chip 8, 227–237 (2008)
J. Wang, L. Ren, L. Li, W. Liu, J. Zhou, W. Yu, D. Tong, S. Chen, Microfluidics: a new cosset for neurobiology. Lab Chip 9, 644–652 (2009)
Y. Xia, G.M. Whitesides, Soft lithography. Annu. Rev. Mater. Sci. 28(1), 153–184 (1998)
Acknowledgments
The authors thank Barbara Smoody for her expert technical assistance, and acknowledge funding from the National Multiple Sclerosis Society (MS Center Grant CA 1055-A-3); CF is supported by a postdoctoral fellowship from the National Multiple Sclerosis Society. ACB is supported by a Graduate Research Fellowship from the National Science Foundation. This work is based upon work supported by the STC Program of the National Science Foundation under Agreement No. ECS-9876771, and was performed in part at the Cornell NanoScale Facility, which is supported by the National Science Foundation (Grant ECS-0335765).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbati, A.C., Fang, C., Banker, G.A. et al. Culture of primary rat hippocampal neurons: design, analysis, and optimization of a microfluidic device for cell seeding, coherent growth, and solute delivery. Biomed Microdevices 15, 97–108 (2013). https://doi.org/10.1007/s10544-012-9691-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-012-9691-2