Abstract
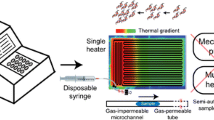
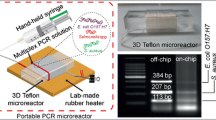
We show the design, development and assessment of disposable, biocompatible, fully plastic microreactors, which are demonstrated to be highly efficient for genomic analyses, such as amplification of DNA, quantitative analyses in real time, multiplex PCR (both in terms of efficiency and selectivity), as compared to conventional laboratory equipment for PCR. The plastic microreactors can easily be coupled to reusable hardware, enabling heating/cooling processes and, in the case of qPCR applications, the real-time detection of the signal from a suitable fluorescent reporter present in the reaction mixture during the analysis. The low cost production of these polymeric microreactors, along with their applicability to a wide range of biochemical targets, may open new perspectives towards practical applications of biochips for point of care diagnostics.



Similar content being viewed by others
Abbreviations
- PCR:
-
Polymerase Chain Reaction
- qPCR:
-
quantitative PCR
- PDMS:
-
Polydimethylsiloxane
- PMMA:
-
Polymethyl-methacrylate
- BSA:
-
Bovine serum albumin
- PEG:
-
Polyethylene-glycol
References
P. Belgrader, S. Young, B. Yuan, M. Primeau, L.A. Christel, F. Pourahmadi, M.A. Northrup, Anal. Chem. 73, 286 (2001). doi:10.1021/ac000905v
S. Bhattacharya, A. Datta, J.M. Berg, S. Gangopadhyay, J. Microelectromech. Syst. 14, 591 (2005). doi:10.1109/JMEMS.2005.844746
D.C. Duffy, J.C. McDonald, O.J.A. Shueller, G.M. Whitesides, Anal. Chem. 70, 4974 (1998). doi:10.1021/ac980656z
B.C. Giordano, E.B. Copeland, J.P. Landers, Electrophoresis 22, 34 (2001). doi:10.1002/1522-2683(200101)22:2<334::AID-ELPS334>3.0.CO;2-O
S.C. Jacobson, A.W. Moore, J.M. Ramsey, Anal. Chem. 67, 2059 (1995). doi:10.1021/ac00109a026
M.U. Kopp, A.J. de Mello, A. Manz, Science 280, 1046 (1998). doi:10.1126/science.280.5366.1046
D.-S. Lee, S.H. Park, H. Yang, K.-H. Chung, T.H. Yoon, S.-J. Kim, K. Kim, Y.T. Kim, Lab Chip 4, 401 (2004). doi:10.1039/b313547k
P. Neuzil, J. Pipper, T.M. Hsieh, Mol. Biosyst. 2, 292 (2006). doi:10.1039/b605957k
M.A. Northrup, B. Benett, D. Hadley, P. Landre, S. Lehew, J. Richards, P. Stratton, Anal. Chem. 70, 918 (1998). doi:10.1021/ac970486a
K.A. Shaikh, K.S. Ryu, E.D. Goluch, J.-M. Nam, J. Liu, C.S. Thaxton, T.N. Chiesl, A.E. Barron, Y. Lu, C.A. Mirkin, C. Liu, Proc. Natl. Acad. Sci. USA 102, 9745 (2005). doi:10.1073/pnas.0504082102
Y.S. Shin, K. Cho, S.H. Lim, S. Chung, S.-J. Park, C. Chung, D.-C. Han, J.K. Chang, J. Micromech. Microeng. 13, 768 (2003). doi:10.1088/0960-1317/13/5/332
K. Suna, A. Yamaguchi, Y. Ishida, S. Matsuo, H. Misawa, Sensors Actuators B 84, 283 (2002)
Y. Weiping, D. Liqun, W. Jing, M. Lingzhi, Z. Jianbo, Sensors Actuators B 108, 695 (2005)
Y. Xia, G.M. Whitesides, Annu. Rev. Mater. Sci. 28, 153 (1998). doi:10.1146/annurev.matsci.28.1.153
Q. Xiang, B. Xu, R. Fu, D. Li, Biomed. Microdevices 7, 273 (2005). doi:10.1007/s10544-005-6069-8
Q. Xiang, B. Xu, D. Li, Biomed. Microdevices 9, 443 (2007). doi:10.1007/s10544-007-9048-4
P. Yager, T. Edwards, E. Fu, K. Helton, K. Nelson, M.R. Tam, B.H. Weigl, Nature 442, 412 (2006). doi:10.1038/nature05064
Acknowledgements
The authors gratefully acknowledge E. Perrone, E. D’Amone, V. Fiorelli, D. Mangiullo and G. Caredda for the expert technical assistance. This work was supported by the Italian Ministry of Research through MIUR “FIRB” project (RBLA03ER38_001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabella, S., Vecchio, G., Pompa, P.P. et al. Disposable plastic microreactors for genomic analyses. Biomed Microdevices 11, 1289–1295 (2009). https://doi.org/10.1007/s10544-009-9348-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-009-9348-y