Abstract
Residue lignin content and biochemistry are important properties influencing residue decomposition dynamics and native soil C loss through priming. The relative contribution of high lignin residues to soil organic matter (SOM) may be less than previously believed, be more sensitive to soil N status, and may be more sensitive to increased temperature. We examined the role of residue biochemistry, temperature, and soil N on the decomposition dynamics of five crop residues varying in lignin content and composition (corn, sorghum, soybean, sunflower and wheat). We used natural abundance δ13CO2 to quantify residue decomposition and soil priming from a soil previously cropped to wheat-fallow or to corn-millet-wheat at 20 and 30 °C in a laboratory incubation. High lignin residues decomposed more completely than low lignin residues, supporting a new model of SOM formation suggesting high lignin residues have a lower efficiency for stabilizing SOM due to inefficient microbial processing. However, residues with lower residue respiration had greater soil C respiration (soil priming). Residue SG lignin was positively related to residue C respired and H-lignin positively related to soil C respired in all soils and temperatures, resulting in no net lignin chemistry effect on the combined total C respired. Effects of lignin on residue decomposition were most apparent in treatments with lower soil N contents indicating N limitation. Measuring both residue and soil respiration and considering soil N status is important to accurately assess the effects of residue biochemistry on soil organic carbon.
Similar content being viewed by others
Introduction
Crop residue return to the soil is essential for maintaining soil fertility and soil organic carbon (SOC) stocks in agroecosystems (Huggins et al. 1998; Paustian et al. 1997; Follett 2001) and increasing the amount of residue returned to the soil results in greater SOC stocks (Huggins et al. 1998). Climate change scenarios predict an increase in mean annual temperature with an increase in precipitation variability that will likely result in lower SOC stocks in semiarid regions due to decreased residue returned to the soil either from crop water stress (CAST 2011) and decreased crop productivity or from flooding and subsequent crop failure (Follett et al. 2012). Further, SOC decomposition accelerates with increasing temperature (Conant et al. 2011) and could lead to decreased SOC stocks in soils under many agricultural management practices (Follett et al. 2012; Burke et al. 1989). In the U.S. Great Plains, crop management systems are being adopted that increase residue return to the soil. These systems include conversion from a conventionally tilled wheat-fallow to a no-till wheat-corn-millet-fallow rotation that increases SOC in the top 15 cm (Halvorson et al. 1997; Mikha et al. 2010). To maintain current SOC stocks and ensure future soil productivity and agricultural sustainability, it is important to maximize crop residue contribution to SOC.
Lignin, a complex molecule comprising up to 20 % by mass of crop residues (Johnson et al. 2007; Martins et al. 2012), is resistant to degradation by extracellular enzymes (Austin and Ballare 2010). Residue decomposition is generally negatively related to residue lignin content (Hofmann et al. 2009; Yanni et al. 2011) and lignin:N ratio (Melillo et al. 1982; Wang et al. 2004). Crop species vary in the amount, chemical composition, and structural composition of lignins (Hatfield et al. 2009). The relative contribution of guaiacyl (G), syringyl (S), and p-hydroxyphenyl (H) lignin monomers as well as p-coumaric and ferulic acids influence lignin degradation (Yanni et al. 2011; Otto and Simpson 2006; Thevenot et al. 2010) and the subsequent lignin chemistry in soil (Filley et al. 2008; Yanni et al. 2011; Feng et al. 2008; Bahri et al. 2006). Grass species also have p-coumaric and ferulic acids encrusting lignins, hemicelluloses and sugars, providing additional structural complexity and biochemical recalcitrance (Hatfield et al. 2009). Residues with high lignin content and greater lignin structural complexity might be expected to decompose more slowly, and persist longer than would low lignin residues.
Residue decomposition varies with the initial biochemical composition of the residue among other factors, with residues with a greater lignin content frequently decomposing more slowly than those with less lignin (Berg and McClaugherty 2008; Parton et al. 2014). However, the importance of residue quality on the residue decomposition dynamics and subsequent stabilization as soil organic matter (SOM) is currently being debated (Cotrufo et al. 2013; von Lutzow et al. 2006; Gentile et al. 2011; Marschner et al. 2008; Dungait et al. 2012). Traditional models of residue decomposition suggest that chemically recalcitrant residues, those with high lignin contents, decompose more slowly than residues with higher soluble sugar, C and N content. Theoretically, these residues lose little C through decomposition and consequently should have a higher proportion stabilized in the soil as soil organic matter (Johnson et al. 2007; Wang et al. 2004; Berg and McClaugherty 2008) through humification or condensation processes (Kogel-Knabner 2002; Martins et al. 2012). Recently, however, a new model of decomposition has been proposed that higher-quality residues (those with more soluble C and N) have a faster decomposition trajectory initially, but in the long-term can stabilize a greater proportion of added residue as SOM (Gleixner et al. 1999) due to more efficient microbial processing (Cotrufo et al. 2013; Prescott 2010; Martins et al. 2012; Parton et al. 2014). High lignin residues are used inefficiently by the soil microbial community (i.e. a low substrate-use efficiency 8–31 %), resulting in much greater respiration losses per unit C added compared to lower-lignin residues. In the traditional model, high-lignin residues form more new SOM, but in the new model, high-lignin residues contribute proportionally less to new SOM due to greater loss through respiration. These two contrasting residue decomposition models (Berg and McClaugherty 2008; Cotrufo et al. 2013) could lead to opposite recommendations for SOC storage. The first case would suggest field addition of more recalcitrant, lignin-rich residues to promote efficient conversion of added residue to SOC and the second, the application of residues with greater soluble sugar and N content.
Decomposition of residues in soils can increase or suppress native soil respiration after addition, depending on the chemical composition of residue added. Since soil microbes are frequently C limited, they are stimulated by C addition resulting in an increase in soil respiration beyond C addition, or soil ‘priming’ (Kuzyakov et al. 2000; Kuzyakov 2010). Soil microbes can also prime existing SOM in order to acquire N compounds (Fontaine et al. 2003; Craine et al. 2007). The net effect of organic matter additions to soil C balance is likely to be a function of the residue biochemical quality and soil properties and it is important to account for them when evaluating residue decomposition dynamics (Kuzyakov 2010).
The relative importance of residue chemistry, especially lignin, in the total amount and rate of residue decomposition is a function of N, which can both positively and negatively impact residue decomposition (Craine et al. 2007; Hobbie et al. 2012). These effects also differ depending on N source—residue N versus fertilizer N additions. Initial decomposition rate is frequently positively related to residue N content (Hobbie 2005; Adair et al. 2008) since at low substrate N concentrations, microbial substrate use can be N limited (Hobbie et al. 2012). However, N fertilization decreases decomposition of high-lignin litter (Knorr et al. 2005) and decreases the activity of lignolytic enzymes (Frey et al. 2004; Hobbie et al. 2012). In residues that are rich in cellulose, N addition stimulates decomposition rate (Hobbie 2000) and cellulose degrading enzyme activity (Keeler et al. 2009). These effects may be due to shifts toward a N-efficient microbial community (Couteaux et al. 1995), microbial N mining from the residue (Craine et al. 2007), or the toxic build-up of compounds that differentially affect lignin-degrading communities (Fog 1988). Recent studies suggest that interactions between residue chemistry and N also change over time, with N stimulating initial cellulose degradation, but resulting in a larger, slowly decomposing pool and decreased lignin enzyme activity (Hobbie et al. 2012). Litter decomposition studies focus on litter N content, but accounting for soil N is particularly important in agricultural soils where residues are mixed via tillage and N fertility is likely to be enhanced. Depending on the N content of the soil, residues rich in cellulose or in lignin could be expected to decompose more efficiently and subsequently contribute to SOC (Hobbie et al. 2012).
Decomposition of residues that are more biochemically recalcitrant (high lignin content) should be more sensitive to increased temperature than more biochemically labile residues (Bosatta and Agren 1999; Conant et al. 2008; Fierer et al. 2005). Enzyme kinetic theory suggests that more recalcitrant compounds will be decomposed at higher temperatures, since the activation energy needed to decompose them is lower at greater temperatures (Fierer et al. 2005; Conant et al. 2008, 2011). In a 53-days incubation, Fierer et al. (2005) found temperature sensitivity of litter decomposition was dependent on substrate quality with lower quality, or residues with greater lignin content having a greater temperature sensitivity. Under scenarios with increased temperature, residues with greater lignin content may decompose more rapidly due to increased temperature sensitivity compared to residues with a lower lignin content (Fierer et al. 2005). This theory suggests that in agricultural systems, high lignin residues would have poorer conversion to SOM at greater temperatures.
We asked the question, “do residues differing in lignin content and biochemistry decompose in the same way in soils from differing agricultural management practices?” In other words, does residue chemistry impact the efficiency of C stabilization through losses via respiration during decomposition? Many traditional litter decomposition methods use litter bags, physically separating soil and litter. Some laboratory methods of measuring crop residue decomposition have used total (soil + residue) respiration and do not account for varying soil versus residue contributions to respiration between litter qualities. Here, we used natural abundance 13CO2 to track the decomposition of five crop residues (corn, sorghum, soybean, sunflower, and wheat) differing in biochemical composition and lignin biochemistry at two temperatures (20 or 30 °C) in two soils differing in N content (a Weld silt loam soil previously cropped to either wheat-fallow [WF] or to corn-millet-wheat [CMW]). We examine if residue decomposition and soil priming are limited by lignin content and biochemistry.
Materials & methods
Soil & plant residues
To obtain two soils that were similar in soil texture and climatic effects, but differing in C and N content, we sampled soils from two long-term no-till crop rotation trials [wheat-fallow (WF) and corn-millet-wheat (CMW)] at the Central Great Plains Research station near Akron, Colorado in 2011. The WF rotation had been established in 1967 (Smika 1990) and the recent site history is described in detail by Follett et al. (2007) and Halvorson et al. (1997). The CMW soil was located in an alternative crop rotation study initiated in 1990 (Mikha et al. 2010). The soil from both rotations was a Weld silt loam, fine montmorillonitic, mesic aridic Paleustoll (30 % sand, 40 % silt, and 30 % clay) (Follett et al. 2007). Mean annual temperature is 9 °C and mean annual precipitation is 420 mm (Mikha et al. 2010). Bulk field soils were collected from the 0–10 cm depth after removing surface litter. Due to the large quantity of wheat stover remaining in the soil, air dried soils were sieved (850 µm) to remove all recognizable surface litter, root material, and rocks.
Five crop residues were collected to obtain a range in residue biochemical quality and lignin content that was representative of crops grown throughout the region. A supply of standing-dead residues of corn (Zea mays L., C4), sorghum [Sorghum bicolor (L.) Moench, C4], soybean (Glycine max L., C3), sunflower (Helianthus annuus L., C3) and wheat (Triticum aestivum L., C3) were collected after crop harvest to use in the experiment and to approximate litter inputs to fields. Crop residues were dried at 65 °C for 48 h, stems separated from other plant organs and ground with a Wiley mill to pass a 2-mm sieve.
Experimental design
To examine the effects of crop residue quality and soil N on decomposition, we incubated the two soils with each of five residues and a no-residue control at either 20 or 30 °C, for 76 days. The experiment was a complete factorial randomized block design with four replicates (microcosms). For each treatment, each replicate consisted of 100 g soil mixed thoroughly with 0.48 g of plant residue in a 150 ml plastic cup and adjusted to soil bulk density of 1 g cm−3. Soils were then wet to field capacity with 20 g water (Follett et al. 2007; Paul et al. 2011) and maintained at field capacity throughout the entire 76 days incubation. Soils were initially pre-incubated overnight in the refrigerator at 4 °C to allow the soil moisture to equilibrate. The soil samples were placed into mason jars (1.76 L) and capped with lids containing septa for gas sampling. Jars were opened and flushed after gas sampling (see below) to prevent excessive accumulation of CO2 and to maintain the CO2 levels below 5 %. Soil samples, including all remaining residue, were destructively sampled at 76 days, sieved to 2 mm and air dried.
Residue biochemistry analyses
Analysis of lignin is difficult due to its heterogeneous composition (Kogel-Knabner 2002). Pyrolysis–gas chromatography–mass spectrometry (py-GC/MS) is a method of molecular fingerprinting after thermal, non-oxidative fragmentation that has been used to characterize plant lignin and SOC composition (del Rio et al. 2007; Nierop et al. 2001; Stewart et al. 2011). Pyrolysis products result in lignin monomers that can be traced to lignin sources and are similar to the non-methylated compounds isolated using the cupric oxide extraction procedure.
Residue biochemical composition was characterized by pyrolysis–gas chromatography–mass spectrometry at 550 °C (Frontier pyrolyzer—Shimadzu QP-2010SE) with a SHRIX-5 ms column (30 m length × 0.25 mm ID, 0.25 µm film thickness). The initial column temperature was 40 °C with a 1 min hold followed by a 7 °C min−1 ramp to 300 °C and a final 5 min hold. The mass spectrometer detection range was 50–600 m/z with the interface temperature held at 300 °C. Compounds were identified using the NIST 2011 mass spectral library and external standards including guaiacol, vanillin, vanillic acid, acetovanillone, syringol, syringaldehyde, acetosyringone, p-hydroxybenzoic acid, p-hydroxy acetophenone, p-hydroxybenzaldehyde, p-coumaric acid, and ferulic acid to verify pyrolysis products. Relative abundances are expressed as peak area as a proportion of identified peak area (%) with precision 2–7 %. This expression of lignin monomer biochemistry is not quantitative, since samples were not externally calibrated. However, total identified lignin S and G monomers are proportional to total acid unhydrolyzable residue (kg/g dry weight, R2 = 0.868, p = 0.021) (AUR).
Each crop litter was analyzed for chemical composition (water-soluble C and N, soluble sugars, acid detergent fiber (ADF), neutral detergent fiber (NDF), and AUR. Water extractable C and N (DOC, DN) was determined using a liquid CN analyzer (Shimadzu TOC-VCPN; Shimadzu Scientific Instruments, Wood Dale, IL, USA) after 1 g plant litter was mixed with 100 ml water and shaken at 80 rpm for 1 h at room temperature and filtered through a 0.45 μm filter paper. Soluble sugars (fructose, glucose, glucose 6-phosphate, mannose, stachyose and sucrose) were determined fluorometrically using high pressure liquid chromatography (WAT085188, Waters Co., Milford, MA) (Trebbi and McGrath 2004). Briefly, 1 ml of 80 % ethanol was added to 25 mg of sample and shaken overnight (50 rpm) at 40 °C. Samples were centrifuged at 3000 rpm for 10 min to settle the residues. The supernatant was then transferred and dried under N2 gas at 60 °C for 24 h. Samples were sent to the ARS-Sugarbeet and Bean Research Unit in East Lansing, MI for HPLC analyses (Trebbi and McGrath 2004).
Plant tissues were sequentially digested for hemicellulose, cellulose, and lignin content with the ANKOM filter bag method using an ANKOM-200 Fiber Analyzer (http://www.ankom.com/analytical-procedures.aspx). This method sequentially extracts: NDF, ADF, and AUR. A 0.5 g oven dried sample was placed in a filter bag and heat sealed. To determine NDF, the sample in the filter bag was first extracted at 100 °C in a fiber analyzer for 75 min using a neutral detergent solution (20 g of sodium sulphite and 4.0 ml of alpha-amylase). Digested NDF samples were rinsed with hot water and then in acetone before being oven dried for 24 h at 100 °C and weighed to determine NDF. To determine the ADF, NDF samples were then digested using an acid detergent solution (20 g cetyl trimethylammonium bromide in 1L 1 N H2SO4) for 60 min., rinsed and dried as above. Acid unhydrolyzable residue was determined by digesting the ADF samples using 72 % H2SO4 for 3 h. To measure ash content, the oven-dried AUR samples were heated at 550 °C for 2 h in a muffle furnace. Plant residues were analyzed for Mn at the Soil, Water & Plant Testing Lab at Colorado State University using Inductively Coupled Plasma—Atomic Emission Spectroscopy.
Plant and soil analyses
Total C, N content (g kg−1 soil) and δ13C of soil (Table 1) and crop residues (Table 2) were determined before incubation by dry combustion using a continuous flow Europa Scientific 20–20 stable isotope analyzer interfaced with Europa Scientific ANCA-NT system Solid/Liquid Preparation Module (Europa Scientific, Crewe Cheshire, UK—Sercon Ltd.). Analytical laboratory plant and soil standards calibrated to Sucrose ANU (δ13C = −10.4, C = 42 %) were used to calibrate each run and to verify instrument performance every 10 samples. The analytical soil standard was 2.44 + 0.02 %C, 0.19 + 0.02 %N, −18.49 + 0.22 δ13C and the plant standard 44.67 + 0.48 %C, 2.53 + 0.02 %N and −12.15 + 0.195 δ13C. Samples were run in duplicate with an analytical error less than 0.05 for plants and 0.35 for soils.
The NO3 −–N and NH4 +–N of the soil before and after incubation were measured by extraction from the soil with 1M Potassium Chloride (KCl) using a 1:5 soil/solution ratio and analyzed using a Lachat 8000 Quik Chem Flow Injector Autoanalyzer (Lachat Instruments, Loveland, CO, USA). Soil pH was measured in 1:1 soil water mixture with a Beckman PHI 45 digital pH meter (USDA/NRCS 2004).
Respiration and δ13CO2
The CO2 concentration was measured periodically (1, 2, 3, 6, 9, 15, 24, 34, 48, 62, and 76 days) using a gas chromatograph (GC) (Varian 3800, Varian Inc., Palo Alto, CA), equipped with a thermal conductivity detector according to the method of Halvorson et al. (2010). After mixing the head space of the incubation jar using a 30 ml syringe, a 20 ml subsample was injected into a 12 ml pre-evacuated Exetainer vial (Labco limited High Wycombe, Buckinghamshire, UK).
The δ13C of the respired CO2 was measured at 15 day intervals using a Micromass VG Optima mass spectrometer (Micromass UK Ltd., Manchester UK). The δ13C was calculated using the equation
where R sample is the ratio of δ13C/δ12C in the respired CO2 and R standard is the ratio of δ13C/δ12C international Pee Dee Belemnite (PDB). Residue-derived respiration (CO2−13C, Q r ,) was calculated using the equation:
where Q t , Q r , Q s , are the respired CO2–C (mg C kg−1 soil) and δt, δr, δs, are its isotopic composition of the total, residue, and soil-respired CO2, respectively. We partitioned soil- and residue-derived respiration using the stable isotope mixing model approach (Balesdent and Balabane 1992):
where δmix = δ13CO2–C at time t, δcontrol = δ13CO2 of the no residue soil; δr = δ13C of the added residue. Error of the Fs was calculated using the Phillips and Gregg (2001) model. Error between measured laboratory replicates was greater than the calculated error from Phillips and Gregg (2001), so we present our measured variability here. Residue-derived CO2–C was calculated by multiplying the above F ratio by the measured CO2–C. Soil-derived respiration was determined as the difference between total C respired and residue-derived respiration. Residue decomposition was expressed as the total residue C added—cumulative residue-derived C respired for each day.
Decomposition model fits
A 2-pool decomposition model was used to model residue decomposition as a function of t, time (Paul 2007).
The residue pools were partitioned into a rapidly decomposing or active pool (C a) and a passive, or slowly decomposing pool (100 − C a). C t is the percentage of the initial residue C at time t. C a is the percentage of C remaining in the active pool, k a (d−1) is the decomposition rate for C a, and k p (d−1) is the decomposition rate of the passive pool (100 − C a). The percentage of residue C remaining will be between 100 and 0. During decomposition there is a possibility of transformation of the labile pool into more recalcitrant materials due to microbial activity, but this model does not consider these transformations.
Statistical analysis
Cumulative residue-derived C values were assessed at the end of the incubation in PROC GLM (SAS 9.3, Cary, NC) using soil, residue addition and temperature as main effects. Treatments were considered significantly different at a 0.05 level. The 2-pool decomposition model was fit to each replicate using PROC NLIN. Parameter estimates k a and k p were evaluated using PROC GLM with main effects of soil, residue addition and temperature. Stepwise linear regression modeling was done using PROC REG. Specific contrasts between soils within each temperature and residue were run using the lsmestimate statement.
Results
Residue chemical composition
The five residues had a wide range in chemical composition, despite a rather narrow C:N content (81–153) (Table 2). Total sugar content ranged from 8.98 to 233.52 g kg−1 dry wt and AUR from 73 to 179 g kg−1 dry wt. Corn had the greatest NDF content (407 g kg−1 dry wt) and sunflower the greatest ADF (424 g kg−1 dry wt). The DOC values ranged from 58 g kg−1 dry wt for sunflower to 410 g kg−1 dry wt for sorghum. Residue Mn ranged from 1.2 to 2.4 g kg−1 dry weight.
Lignin biomarker biochemistry determined by py-GC/MS (Table 3) was quite distinct between the C3 (soybean, sunflower, wheat) and C4 (corn, sorghum) plant species, reflecting known differences in lignin structure. The C3 plants had a greater G + S content (SG), comprising 36.3–46.6 % of the monomers detected. Predominant G units were 4-ethoxy-3-hydroxy-benzaldehyde, guaiacol, and coniferyl alcohol [4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol]. The predominate S-units were syringol, 4-vinylsyringol, and methoxyeugenol. The C4 species had higher relative abundance of H-units, comprising more than 30 % of the monomers detected. The compound 4-vinylphenol, a typical pyrolysis product of p-coumaric acid (del Rio et al. 2007), comprised 25.9 for sorghum and 30.2 relative abundance for corn compared to the C3 plants (0, 0.9, and 8.4 for sunflower, soybean and wheat, respectively). All residues also contained between 6 and 9 % of 4-vinylguaiacol, a pyrolysis product of ferulic acid (del Rio et al. 2007).
Soil C and N
Long-term CMW rotation produced soil with greater C and N content compared to WF (Table 1). The CMW soil had 1.4 times more soil C and N than WF (18.5 vs. 12.9 g kg soil−1 and 1.69 vs. 1.13 g kg soil−1, respectively) (Table 1). Nitrate and ammonium concentration was also higher in the CMW soil. Initial soil NH4 + concentration was 2.5 times and NO3 − concentration was 4.1 times higher in the CMW soil compared to the WF soil, respectively (Fig. 1). Over the entire incubation, all treatments and residue additions immobilized NO3 −–N compared to the no residue control, except for CMW-30 (Fig. 1a). Increasing the temperature from 20 to 30 °C resulted in 2.3 times more NO3 −–N in the CMW soil (Fig. 1a) and 1.9 times more NO3 −–N in the WF soil. Ammonium decreased throughout the incubation compared to the initial content (Fig. 1b) and was significantly greater in the no-residue control CMW-30 treatment compared to all residue additions (Fig. 1b).
Residue remaining
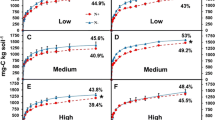
Residue remaining, averaged across temperature and soil, decreased with increasing residue lignin content (R2 = 0.753, Table 4). Residues with the greatest SG content had the least residue C remaining averaged across temperature and soil (soybean (47.1 %) < sunflower (50.8 %) < wheat (55.7 %) < sorghum (58.1 %) < corn (64.5 %) (p < 0.0001, Fig. 2). However, this order varied across soil and temperature treatments. There was no significant effect of soil averaged across residues, but increasing the temperature from 20 to 30 °C decreased residue C remaining an average 8 % (Table 4).
The same residue was not consistently decomposed in each soil and this effect was dependent on temperature (residue × soil × temperature interaction, p = 0.018) (Table 4). At 20 °C, the high lignin residues (higher SG content), soybean and sunflower, had less residue C remaining in the CMW soil compared to WF (p = 0.002, p < 0.0001). However at 30 °C, the low-lignin (S + G residues), corn and sorghum, had more residue C remaining in the CMW compared to the WF soil (Table 4).
Decomposition rates
Averaged across soil and temperature, the decomposition rate k a increased with increasing residue lignin content, corn (0.82 %C d−1 × 10), wheat (0.87 %C d−1 × 10), sunflower (0.91 %C d−1 × 10) and soybean (1.20 %C d−1 × 10) with the exception of sorghum (1.34 %C d−1 × 10). The WF soil on average had a slightly greater k a compared to CWM (1.06 and 1.00 %C d−1 × 10, respectively). Increasing the temperature from 20 to 30 °C increased k a from 0.94 to 1.12 %C d−1 × 10 (Table 4). At 20 °C, the low lignin residues (sorghum and corn) had significantly decreased k a in the CWM compared to the WF soil and high-lignin soybean had increased k a in CWM. At 30 °C, only the k a of C was significantly lower in the CWM compared to WF soil.
Decomposition rates of k p averaged across soil and temperature also increased with increasing residue lignin content; corn (2.1 %C d−1 × 103), sorghum (2.5 %C d−1×103), wheat (3.7 %C d−1 × 103), sunflower (4.1 %C d−1 × 103) and soybean (5.6 %C d−1 × 103). Averaged across residue and temperature, the WF soil had a slightly greater k p compared to CWM (4.1 and 3.1 %C d−1 × 103, respectively). Increasing the temperature from 20 to 30 °C decreased k p from 4.0 to 3.2 (Table 4). At 20 °C, the lower-lignin residues had a faster k p in the WF compared to CMW soil, while the high-lignin residues (sunflower and soybean) had greater k p in the CWM soil, although this was only significant in soybean. At 30 °C, k p was lower for all residues in the CWM compared to the WF soil, except sunflower.
Lignin biochemistry explains decomposition dynamics
Residue lignin G, S, and H content effectively described contrasting residue decomposition dynamics across soil and temperatures (Table 5). The H-type lignins were strongly positively correlated with residue C remaining in both soils at 20 °C (WF R2 = 0.90 and CMW R2 = 0.92). Residue C remaining at 30 °C negatively correlated to G-type lignins (WF R2 = −0.48 and CMW R2 = −0.68) (Table 5).
All lignin metrics were positively related to k p (Table 5). At 30 °C k p was positively correlated to G-type lignins (WF R2 = 0.59 and CMW R2 = 0.77) and at 20 °C correlated with SG lignin (R2 = 0.71) in WF and AUR (R2 = 0.91) in CMW soil. However, k a was positively related to G lignin only in the CMW soil (R2 = 0.97) and to DOC:DN ratio in all other treatments. Residue Mn was not related to decomposition dynamics in any of the temperature or soil treatments in contrast to Berg (2014).
Residue priming of SOC
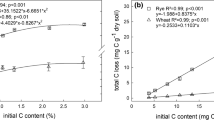
Residue addition stimulated soil C decomposition in all treatments and comprised between 33 and 59 % of total respired C. Residue lignin G, S, and H content described the partitioning of respiration from either the soil or residue. Residue SG lignin was positively related to residue C respired while H-lignin was positively related to soil C respired in all soils and temperatures. Cumulative residue C respired was positively related to py-GC/MS total SG and G relative abundance in all soils × temperature combinations (Fig. 3a, b; Table 5). The slope of the relationship was greater at 20 °C compared to 30 °C. Soil-derived C was positively related to H-type lignin content for all soils and temperatures (Fig. 3c).
Within each soil-temperature treatment, residue and soil-derived C were strongly related (Fig. 4). Although residue lignin G, S, and H content influenced the relative proportion of residue versus soil-derived C respired, there was no effect of lignin chemistry on total C respired. Within treatment total C respired was weakly related to DN in the WF soil and ash in the CMW soil (Table 5).
Discussion
Residue lignin content increased total residue decomposed
Residues with greater lignin content decomposed more completely than lower lignin residues, supporting a new model of SOM formation. In this model, lignin-rich residues are decomposed inefficiently resulting in greater loss through respiration and contribute proportionally less to new SOM (Cotrufo et al. 2013; Prescott 2010; Martins et al. 2012; Parton et al. 2014). In all soils, greater residue SG and G lignin content correlated with greater residue decomposition (residue-derived C loss, Fig. 3a, b). This directly contrasts with traditional models of decomposition which describe decomposition patterns in which lignin-rich residues decompose slowly and have the greatest proportion of residue remaining (Berg and McClaugherty 2008) which has been interpreted and frequently modeled as contributing to SOM formation (Cotrufo et al. 2013; Parton et al. 2014).
Greater decomposition of high lignin residues could be a function of low microbial substrate-use efficiency (SUE)–the proportion of assimilated substrates used for growth and enzyme production compared to respiration (Cotrufo et al. 2013). Due to the complexity of lignin-cellulose, microbial substrate use efficiency for lignin is low (between 8 and 31 %, Cotrufo et al. 2013; Bahri et al. 2008), decreasing with increasing lignin content (Lekkerkerk et al. 1990) and increasing substrate C:N ratio (Sinsabaugh et al. 2013). Lignin-protected cellulose requires initial depolymerization by extracellular enzymes which are energetically costly, resulting in greater respiration. The SUE of low-lignin litter is higher, resulting in proportionally less C respired, leaving a greater quantity of plant-derived C remaining. Substrates such as glucose can be used as efficiently as 73 % (Dijkstra et al. 2011). In this study, the high lignin residues soybean and sunflower had the least residue-C remaining in all soils from greater respiration losses per unit C added compared to lower-lignin residues. This implies that short-term increases in soil residue-derived C can be stimulated by residues with low lignin content (Gentile et al. 2011; Martens 2002).
Residue lignin chemistry
Plants vary in their lignin biochemistry, with angiosperms containing a mix of guaiacyl (G), syringyl (S), p-hydroxyphenyl (H) lignin monomers. Grasses also have lignocellulose complexes that are acetylated or esterified by H-type lignin acids, p-coumaric and ferulic acids (del Rio et al. 2007; Hatfield et al. 2009). We found that both corn and sorghum had a large proportion of the pyrolysis product of p-coumaric acid (del Rio et al. 2007), 4-vinyl phenol (30.2 and 25.8 %). This agrees with other work that found greater p-coumaric acid concentrations for sorghum (30.8 mg p-coumaric acid g−1 cell wall) and corn (38.7 mg p-coumaric acid g−1 cell wall) compared to other C3 and C4 grasses (Hatfield et al. 2009). The larger concentration of these compounds reflects the greater amount of acid-linked lignocellulose in these C4 grasses, which could influence digestibility and decomposition (Hatfield et al. 2009).
Due to cell wall structural differences between residues, SG lignins and H lignins were effective in describing contrasting residue decomposition dynamics. The H-lignin pyrolysis derivatives of p-coumaric and ferulic acids (4-vinyl phenol and 4 vinyl guaiacol) were positively correlated with residue total sugars (r = 0.61, p < 0.0001) and NDF (r = 0.73, p < 0.0001) and positively correlated to residue C remaining at 20 °C. In contrast, residue G lignin content was positively related to AUR (r = 0.93, p < 0.0001) and positively related to residue C respired in all soils and temperatures. Residue G content, determined by CuO has been linked to resistance to decomposition due to its more condensed structure and/or to the preferential decomposition of other lignin subunits, resulting in a greater remaining G lignin concentration compared to other lignin monomers (Ertel and Hedges 1984; Dignac et al. 2005; Yanni et al. 2011). However, we do not observe this apparent recalcitrance here.
Soil N status determines residue chemistry effects
Residue decomposition is a function of both inherent litter N content and N addition and has been shown to both stimulate and inhibit decomposition depending on N source and residue lignin content (Craine et al. 2007; Hobbie et al. 2012). Decomposition studies generally have not evaluated inherent soil N effects on residue decomposition. However, we observed both inhibition and stimulation of decomposition in this study, depending on treatment, soil N content and residue chemistry. These observed effects are similar to those noted in the literature for litter N content and inorganic N fertilizer addition.
Soil incubation created treatments with varying levels of soil inorganic N, based on initial inorganic soil N content and mineralization of SOM and increased from WF-20 < CMW-20 ≤ WF-30 < CWM-30. These conditions created two distinct effects of residue decomposition over varying soil N contents. Decomposition of lignin-rich residues was higher in soils with greater N content (CMW compared to WF), indicating reduced N limitation in those treatments at 20 °C. Residue addition immobilized N compared to the no-residue treatment. Increased residue decomposition with greater inherent litter N has been observed in other studies (Cornwell et al. 2008), where the addition of N releases C limitation of decomposition (Hobbie et al. 2012). In other studies though, high lignin litter decomposed more slowly due to decreased activity of lignolytic enzymes (Frey et al. 2004; Hobbie et al. 2012). In a meta-analysis, Knorr et al. (2005) found N addition generally decreased decomposition, but the effects were highly variable.
A further increase in soil N had the opposite effect at 30 °C; where decomposition was lower at higher soil N contents (CMW compared to WF) for the low-lignin residues, suggesting an inhibition at higher soil N contents. Nitrogen immobilization was observed with residue addition in the WF, but in the CMW soil decomposition appeared not to be N limited. Hobbie et al. (2012) found that externally supplied N resulted in a faster initial decomposition rate with more cellulose degrading enzyme activity but resulted in a larger slowly decomposing litter pool. We observed a larger residue pool remaining for the low-lignin residues in the higher N soil. This observation is consistent with increased microbial substrate use efficiency of low-lignin residues applied in a high N soil, resulting in a larger quantity of residue C remaining (sensu Cotrufo et al. 2013).
Soil C priming and residue lignin chemistry
Residue addition can stimulate soil priming by providing a labile C source for the soil microbial biomass (Kuzyakov 2010) and was observed in all treatments. Soil C respired was strongly positively related to H-lignin content (pyrolysis products of p-coumaric and ferulic acids) (Fig. 3c). Residues with greater H-lignin content represented the low-lignin samples, sorghum and corn. Since p-coumaric and ferulic acids link hemicelluloses and sugars, this may have little to do with the inherent digestibility of p-coumaric and ferulic acids, and more to do with the fact they integrate the high sugar and hemicellulose content of those residues into a single variable. Indeed, the H-lignin content was positively correlated with residue total sugars (r = 0.61, p < 0.0001) and NDF (r = 0.73, p < 0.0001).
Residue lignin chemistry was important in partitioning residue- versus soil-derived respiration. High-lignin residues (S + G) had a greater proportion of residue-derived respiration, while low-lignin residues had proportionally more soil-derived respiration. However, within each soil-temperature treatment, residue and soil-derived C were strongly related (Fig. 4). Despite lignin SG and H content effectively predicting both residue- and soil-derived respiration separately, when combined, total C respired over the course of the incubation was not related to any residue quality parameter (Table 5). Instead, total residue respired was weakly related to DN in the WF soil and ash in the CWM soil, suggesting N limitation or other limitations to total C lost from the system. Considering both residue- and soil-C losses together is important to accurately determine residue effects on total soil C.
Conclusion
Residues with greater lignin content had less residue C remaining indicating that high lignin residues in this study contribute proportionally less to new SOM due to greater loss through microbial respiration. Our data are consistent with new models suggesting lignin-rich residues decompose slower, but more completely than residues with low lignin content (Cotrufo et al. 2013; Prescott 2010). However, lower residue respiration was strongly related to larger soil C loss (i.e. soil priming) for each temperature × soil combination such that low-lignin residues had a large percentage of soil C respired. Although residue lignin chemistry was important in the partitioning of respiration between residue and soil, total C respired was not related to residue lignin biochemistry, suggesting an overall N limitation to the total C lost from the system.
Effects of lignin on residue decomposition were most apparent in treatments with lower soil N contents indicating N limitation. The differential effect of residue biochemistry with increasing soil N may be due to increasing microbial substrate use efficiency and agrees with recent findings that lignin is not particularly recalcitrant and rarely preserved intact in soils over the long-term. High lignin residues, when added as a soil amendment, will actually have poorer conversion to SOM than those with lower lignin contents, especially under low soil N conditions. However, accounting for soil C loss in addition to SOC formation from residue decomposition will be important to accurately predict the net residue impacts on SOC.
Abbreviations
- SOC:
-
Soil organic carbon
- C:
-
Carbon
- N:
-
Nitrogen
- WF:
-
Wheat-fallow
- CMW:
-
Corn-millet-wheat
- G:
-
Guaiacyl
- S:
-
Syringyl
- H:
-
p-hydroxyphenyl
- SG:
-
Guaiacyl + syringyl
- SOM:
-
Soil organic matter
- Py–GC/MS:
-
Pyrolysis–gas chromatography–mass spectrometry
- ADF:
-
Acid detergent fiber
- NDF:
-
Neutral detergent fiber
- AUR:
-
Acid unhydrolyzable residue
- DOC:
-
Dissolved organic C
- DN:
-
Dissolved N
- SUE:
-
Substrate-use efficiency
References
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Change Biol 14(11):2636–2660
Austin AT, Ballare CL (2010) Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc Natl Acad Sci USA 107(10):4618–4622
Bahri H, Dignac MF, Rumpel C, Rasse DP, Chenu C, Mariotti A (2006) Lignin turnover kinetics in an agricultural soil is monomer specific. Soil Biol Biochem 38(7):1977–1988
Bahri H, Rasse DP, Rumpel C, Dignac MF, Bardoux G, Mariotti A (2008) Lignin degradation during a laboratory incubation followed by 13C isotope analysis. Soil Biol Biochem 40(7):1916–1922
Balesdent J, Balabane M (1992) Maize root-derived soil organic carbon estimated by natural 13C abundance. Soil Biol Biochem 24(2):97–101
Berg B (2014) Decomposition patterns for foliar litter—a theory for influencing factors. Soil Biol Biochem 78:222–232
Berg B, McClaugherty C (2008) Plant litter decomposition, humus formation, carbon sequestration. Springer, Berlin
Bosatta E, Agren GI (1999) Soil organic matter quality interpreted thermodynamically. Soil Biol Biochem 31(13):1889–1891
Burke IC, Yonker CM, Parton WJ, Cole CV, Flach K, Schimel DS (1989) Texture, climate, and cultivation effects on soil organic-matter content in US grassland soils. Soil Sci Soc Am J 53(3):800–805
CAST report (2011) Carbon sequestration and greenhouse gas fluxes in agriculture: Challenges and opportunities In: Carbon sequestration and greenhouse gas fluxes in agriculture: Challenges and opportunities. Council for Agricultural Science and Technology Issue Paper. p 112
Conant RT, Drijber RA, Haddix ML, Parton WJ, Paul EA, Plante AF, Six J, Steinweg JM (2008) Sensitivity of organic matter decomposition to warming varies with its quality. Glob Change Biol 14(4):868–877
Conant RT, Ryan MG, Ågren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvönen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Megan Steinweg J, Wallenstein MD, Martin Wetterstedt JÅ, Bradford MA (2011) Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob Change Biol 17(11):3392–3404
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11(10):1065–1071
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (mems) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19(4):988–995
Couteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and liter quality. Trends Ecol Evol 10(2):63–66
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88(8):2105–2113
del Rio JC, Gutierrez A, Rodriguez IM, Ibarra D, Martinez AT (2007) Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FTIR. J Anal Appl Pyrol 79(1–2):39–46
Dignac MF, Bahri H, Rumpel C, Rasse DP, Bardoux G, Balesdent J, Girardin C, Chenu C, Mariotti A (2005) Carbon-13 natural abundance as a tool to study the dynamics of lignin monomers in soil: an appraisal at the Closeaux experimental field (France). Geoderma 128(1–2):3–17
Dijkstra P, Thomas SC, Heinrich PL, Koch GW, Schwartz E, Hungate BA (2011) Effect of temperature on metabolic activity of intact microbial communities: evidence for altered metabolic pathway activity but not for increased maintenance respiration and reduced carbon use efficiency. Soil Biol Biochem 43(10):2023–2031
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Change Biol 18(6):1781–1796
Ertel JR, Hedges JI (1984) The lignin component of humic substances–distribution among soil and sedimentary humic, fulvic, and base-insoluble fractions. Geochim Cosmochim Acta 48(10):2065–2074
Feng XJ, Simpson AJ, Wilson KP, Williams DD, Simpson MJ (2008) Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat Geosci 1(12):836–839
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86(2):320–326
Filley TR, Boutton TW, Liao JD, Jastrow JD, Gamblin DE (2008) Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J Geophys Res 113:G03009. doi:10.1029/2007JG000564
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
Follett RF (2001) Soil management concepts and carbon sequestration in cropland soils. Soil Tillage Res 61(1–2):77–92
Follett RF, Paul EA, Pruessner EG (2007) Soil carbon dynamics during a long-term incubation study involving C-13 and C-14 measurements. Soil Sci 172(3):189–208
Follett RF, Stewart CE, Pruessner EG, Kimble JM (2012) Effects of climate change on soil carbon and nitrogen storage in the US Great Plains. J Soil Water Conserv 67(5):331–342
Fontaine S, Mariotti A, Abbadie L (2003) The priming effect of organic matter: a question of microbial competition? Soil Biol Biochem 35(6):837–843
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For Ecol Manage 196(1):159–171
Gentile R, Vanlauwe B, Six J (2011) Litter quality impacts short- but not long-term soil carbon dynamics in soil aggregate fractions. Ecol Appl 21(3):695–703
Gleixner G, Bol R, Balesdent J (1999) Molecular insight into soil carbon turnover. Rapid Commun Mass Spectrom 13(13):1278–1283
Halvorson AD, Vigil MF, Peterson GA, Elliott ET (1997) Long-term tillage and crop residue management study at Akron, CO. In: Paul EA, Paustian K, Elliot ET, Cole CV (eds) Soil organic matter in temperate agroecosystems. CRC Press, New York, pp 361–370
Halvorson AD, Del Grosso SJ, Alluvione F (2010) Tillage and inorganic nitrogen source effects on nitrous oxide emissions from irrigated cropping systems. Soil Sci Soc Am J 74(2):436–445
Hatfield RD, Marita JM, Frost K, Grabber J, Ralph J, Lu FC, Kim H (2009) Grass lignin acylation: p-coumaroyl transferase activity and cell wall characteristics of C3 and C4 grasses. Planta 229(6):1253–1267
Hobbie SE (2000) Interactions between litter lignin and nitrogenitter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3(5):484–494
Hobbie SE (2005) Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8(6):644–656
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82(3):389–405
Hofmann A, Heim A, Christensen BT, Miltner A, Gehre M, Schmidt MWI (2009) Lignin dynamics in two C-13-labelled arable soils during 18 years. Eur J Soil Sci 60(2):250–257
Huggins DR, Buyanovsky GA, Wagner GH, Brown JR, Darmody RG, Peck TR, Lesoing GW, Vanotti MB, Bundy LG (1998) Soil organic C in the tallgrass prairie-derived region of the corn belt: effects of long-term crop management. Soil Tillage Res 47(3–4):219–234
Johnson JM-F, Barbour NW, Weyers SL (2007) Chemical composition of crop biomass impacts its decomposition. Soil Sci Soc Am J 71(1):155–162
Keeler B, Hobbie S, Kellogg L (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12(1):1–15
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86(12):3252–3257
Kogel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34(2):139–162
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42(9):1363–1371
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32(11–12):1485–1498
Lekkerkerk L, Lundkvist H, Agren G, Ekbohm G, Bosatta E (1990) Decomposition of heterogeneous substrates; An experimental investigation of a hypothesis on substrate and microbial properties. Soil Biol Biochem 22:161–167
Marschner B, Brodowski S, Dreves A, Gleixner G, Gude A, Grootes PM, Hamer U, Heim A, Jandl G, Ji R, Kaiser K, Kalbitz K, Kramer C, Leinweber P, Rethemeyer J, Schäffer A, Schmidt MWI, Schwark L, Wiesenberg GLB (2008) How relevant is recalcitrance for the stabilization of organic matter in soils? J Plant Nutr Soil Sci 171(1):91–110
Martens DA (2002) Identification of phenolic acid composition of alkali-extracted plants and soils. Soil Sci Soc Am J 66(4):1240–1248
Martins MR, Angers DA, Corá JE (2012) Co-accumulation of microbial residues and particulate organic matter in the surface layer of a no-till Oxisol under different crops. Soil Biol Biochem 50:208–213
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Mikha MM, Benjamin JG, Vigil MF, Nielson DC (2010) Cropping intensity impacts on soil aggregation and carbon sequestration in the central great plains. Soil Sci Soc Am J 74(5):1712–1719
Nierop KGJ, van Lagen B, Buurman P (2001) Composition of plant tissues and soil organic matter in the first stages of a vegetation succession. Geoderma 100(1–2):1–24
Otto A, Simpson MJ (2006) Evaluation of CuO oxidation parameters for determining the source and stage of lignin degradation in soil. Biogeochemistry 80(2):121–142
Parton WJ, Del Grosso SJ, Plante AF, Adair EC (2014) The dynamics of soil organic matter and nutrient cycling. In: Paul EA (ed) Soil microbiology, ecology and biochemistry. Academic Press, San Diego
Paul EA (ed) (2007) Soil microbiology, ecology, and biochemistry. Academic Press, Amsterdam
Paul EA, Follett RF, Haddix M, Pruessner E (2011) Soil N dynamics related to soil C and microbial changes during long-term incubation. Soil Sci 176(10):527–536
Paustian K, Andrén O, Janzen HH, Lal R, Smith P, Tian G, Tiessen H, Van Noordwijk M, Woomer PL (1997) Agricultural soils as a sink to mitigate CO2 emissions. Soil Use Manag 13:230–244
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127(2):171–179
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101(1–3):133–149
Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A (2013) Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol Lett 16(7):930–939
Smika DE (1990) Fallow management-practices for wheat production in the Central Great Plains. Agron J 82(2):319–323
Stewart CE, Neff JC, Amatangelo KL, Vitousek PM (2011) Vegetation effects on soil organic matter chemistry of aggregate fractions in a Hawaiian forest. Ecosystems 14(3):382–397
Thevenot M, Dignac MF, Rumpel C (2010) Fate of lignins in soils: a review. Soil Biol Biochem 42(8):1200–1211
Trebbi D, McGrath JM (2004) Fluorometric sucrose evaluation for sugar beet. J Agric Food Chem 52(23):6862–6867
USDA/NRCS Soil survey laboratory methods manual No. 42, Version 4.0, November 2004 Rebecca Burt, Editor
von Lutzow M, Kogel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57(4):426–445
Wang WJ, Baldock JA, Dalala RC, Moody PW (2004) Decomposition dynamics of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Biol Biochem 36(12):2045–2058
Yanni SF, Whalen JK, Simpson MJ, Janzen HH (2011) Plant lignin and nitrogen contents control carbon dioxide production and nitrogen mineralization in soils incubated with Bt and non-Bt corn residues. Soil Biol Biochem 43(1):63–66
Acknowledgments
The authors thank Elizabeth Pruessner, Erin Grogan, Mary Smith, Brad Floyd, Robert D’Adamo, Travis Delorean, and many students for their assistance and analytical support in collecting, processing, and analyzing the soil and plant samples and data reported herein and the Central Great Plains Research Staff for maintaining these long-term plots. The authors thank anonymous reviewers and the editor for their thoughtful comments and suggestions to improve the manuscript. This publication is based upon work supported by the Agricultural Research Service under the ARS GRACEnet Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Melany Fisk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stewart, C.E., Moturi, P., Follett, R.F. et al. Lignin biochemistry and soil N determine crop residue decomposition and soil priming. Biogeochemistry 124, 335–351 (2015). https://doi.org/10.1007/s10533-015-0101-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0101-8