Abstract
In arid regions, spring-fed habitats are frequently the only year-round source of surface water and are essential habitats for aquatic organisms and primary water sources for terrestrial animals and human settlements. While these habitats have been relatively well-studied in some regions, those of the southern Sonoran Desert have received little attention. In 2008 and 2009, we documented the biodiversity of aquatic animals at 19 sites across three arid mountain ranges in Sonora, Mexico, characterized macrohabitat types, examined seasonal variation in aquatic invertebrate communities, and explored the effects of an exotic fish (tilapia) on native communities. We documented >220 aquatic animal species, including several new species and range extensions for others. Macrohabitat type (oasis, tinaja, riffle, and seep) was more important than geographic location in structuring aquatic invertebrate communities at the scale of our study area (~9,000 km2). We found little evidence of predictable seasonal variation in invertebrate communities, despite dramatic hurricane-induced flooding. Aquatic vertebrates were not diverse across the study region (4 amphibian species and 2 species each of fishes and reptiles), but were often locally abundant. Presence of non-native tilapia at one site was associated with reduced abundances of native leopard frogs and reduced richness and density of native aquatic invertebrates. The most pressing aquatic habitat conservation concerns in the region, as in other deserts, are groundwater withdrawal, unmanaged recreational visitation, and the introduction of exotic species. Spring-fed habitats around the world have been called hotspots of freshwater biodiversity, and those of the Sonoran Desert are no exception.








Similar content being viewed by others
References
Andreu-Soler A, Ruiz-Campos G (2013) Effects of exotic fishes on the somatic condition of the endangered killifish Fundulus lima (Teleostei: Fundulidae) in oases of Baja California Sur, Mexico. Southwest Nat 58(2):192–201
Benqlilou H, Bensaid S (2013) Protection and performance of the ancestral water supply system ‘Khettara’ as a sustainable alternative for arid regions. Water Sci Tech Water Supply 13(6):1452–1462
Bogan MT (2012) Drought, dispersal, and community dynamics in arid-land streams. Dissertation, Oregon State University, Corvallis
Bogan MT, Lytle DA (2007) Seasonal flow variation allows ‘time-sharing’ by disparate aquatic insect communities in montane desert streams. Freshw Biol 52:290–304
Bogan MT, Lytle DA (2011) Severe drought drives novel community trajectories in desert stream pools. Freshw Biol 56:2070–2081
Bogan MT, Findley LT, Endersen EF (2009) Geographic distribution: Tantilla yaquia (Yaqui black-headed snake). Herpetol Rev 40(4):458
Box JB, Duguid A, Read RE, Kimber RG, Knapton A, Davis J, Bowland AE (2008) Central Australian waterbodies: the importance of permanence in a desert landscape. J Arid Environ 72:1395–1413
Cantonati M, Fureder L, Gerecke R, Juttner I, Cox EJ (2012) Crenic habitats, hotspots for freshwater biodiversity conservation: towards an understanding of their ecology. Fresh Sci 31(2):463–480
Carrizales, J (2007) Cañón del nacapule: peligra paraíso ecológico. El imparcial (newspaper; Hermosillo, Sonora) http://expresionguaymas.wordpress.com/2007/01/22/canon-del-nacapule-peligra-paraiso-ecologico. Accessed 8 Feb 2014
Collier KJ, Smith B (2006) Distinctive invertebrate assemblages in rockface seepages enhance lotic biodiversity in northern New Zealand. Biodivers Conserv 15:3591–3616
Cox CB, Moore PD (1993) Biogeography: an ecological and evolutionary approach. Blackwell Scientific, London
Custodio E (2002) Aquifer overexploitation: what does it mean? Hydrogeol J 10:245–277
Deacon JE, Williams AE, Williams CD, Williams JE (2007) Fueling population growth in Las Vegas: how large-scale groundwater withdrawal could burn regional diversity. Bioscience 57(8):688–698
Dinger EC, Cohen AE, Hendrickson DA, Marks JC (2005) Aquatic macroinvertebrates of Cuatro Cienegas, Coahuila, Mexico: natives and exotics. Southwest Nat 50(2):237–281
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Leveque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Felger RS (1999) The flora of Cañón de Nacapule: a desert-bounded tropical canyon near Guaymas, Sonora, Mexico. Proc S Diego Soc Nat Hist 35:1–42
Fensham RJ, Silcock JL, Kerezsy A, Ponder W (2011) Four desert waters: setting arid zone wetland conservation priorities through understanding patterns of endemism. Biol Conserv 144:2459–2467
Gallo-Reynoso, JP (2003) Estudio previo justificativo para proponer el establecimiento de la región “Sierra del Aguaje, Bahía de San Francisco e Isla San Pedro Nolasco y sus aguas aledañas” como una nueva Área Natural Protegida. http://www.guaymas.gob.mx/ecologia/reserva.pdf. Accessed 28 Sep 2008
Grismer LL, McGuire JA (1993) The oases of central Baja California, Mexico, part I. A preliminary account of the relict mesophilic herptofauna and the status of the oases. Bull South Calif Acad Sci 92(1):2–24
Hadwen WL, Boon PI, Arthington AH (2012) Aquatic ecosystems in inland Australia: tourism and recreational significance, ecological impacts and imperatives for management. Mar Freshw Res 63(4):325–340
Hershler R, Liu H-P, Landye JJ (2011) New species and records of springsnails (Caenogastropoda: Cochliopidae: Tryonia) from the Chihuahuan Desert (Mexico and United States), an imperiled biodiversity hotspot. Zootaxa 3001:1–32
Jenni-Eiermann S, Almasi B, Maggini I, Salewski V, Bruderer B, Liechti F, Jenni L (2011) Numbers, foraging and refuelling of passerine migrants at a stopover site in the western Sahara: diverse strategies to cross a desert. J Ornithol 152(Supplement 1):113–128
Luja VH, Rodríguez-Estrella R (2010a) Are tropical cyclones sources of natural selection? Observations on the abundance and behavior of frogs affected by extreme climatic events in the Baja California peninsula, Mexico. J Arid Environ 74:1345–1347
Luja VH, Rodríguez-Estrella R (2010b) The invasive bullfrog Lithobates catesbeianus in oases of Baja California Sur, Mexico: potential effects in a fragile ecosystem. Biol Invasions 12:2979–2983
Maezono Y, Kobayashi R, Miyashita T (2005) Direct and indirect effects of exotic bass and bluegill on exotic and native organisms in farm ponds. Ecol Appl 15:638–650
Martens A, Richter O, Suhling F (2010) The relevance of perennial springs for regional biodiversity and conservation. In: Schmiedel U, Jürgens N (eds) Biodiversity in southern Africa, vol 2., Patterns and processes at regional scaleKlaus Hess Publishers, Göttingen & Windhoek, pp 70–74
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach
Mielke PW Jr, Berry KJ (2001) Permutation methods: a distance function approach. Springer, Berlin
Miller RR, Minckley WL, Norris SM (2006) Freshwater fishes of Mexico. University of Chicago Press, Chicago
Murphy NP, Adams M, Guzik MT, Austin AD (2013) Extraordinary micro-endemism in Australian desert spring amphipods. Mol Phylogenet Evol 66:645–653
Nevill JC, Hancock PJ, Murray BR, Ponder WF, Humphreys WF, Phillips ML, Groom PK (2010) Groundwater-dependent ecosystems and the dangers of groundwater overdraft: a review and an Australian perspective. Pac Conserv Biol 16(3):187–208
Pfeiler E, Markow TA (2008) Phylogenetic relationships of leopard frogs (Rana pipiens complex) from an isolated coastal mountain range in southern Sonora, Mexico. Mol Phylogenet Evol 49:343–348
Rackemann SL, Robson BJ, Matthews TG (2013) Conservation values of waterfalls as habitat for lotic insects of western Victoria, Australia. Aquat Conserv Mar Freshw Ecosyst 23:171–178
Rader RB, Keleher MJ, Billman E, Larsen R (2012) History, rather than contemporary processes, determines the variation in macroinvertebrate diversity in artesian springs: the expansion hypothesis. Freshw Biol 57:2475–2486
Recuero E, Martínez-Solano I, Parra-Olea G, García-París M (2006) Phylogeography of Pseudacris regilla (Anura: Hylidae) in western North America, with a proposal for a new taxonomic rearrangement. Mol Phylogenet Evol 39:293–304
Ricciardi A, Rasmussen JB (1999) Extinction rates of North American freshwater fauna. Conserv Biol 13(5):1220–1222
Rodríguez-Estrella R, Blázquez MC, Lobato JM (2005) Avian communities of arroyos and desert oases in Baja California Sur: implications for conservation. In: Cartron JE, Ceballos G, Felger RS (eds) Biodiversity, ecosystems, and conservation in northern Mexico. Oxford University Press, Oxford, pp 334–353
Ruiz-Campos G, Varela-Romero A, Sánchez-Gonzalez S, Camarena-Rosales F, Maeda-Martínez AM, Gonzalez-Acosta AF, Andreu-Soler A, Campos-Gonzalez E, Delgadillo-Rodríguez J (2014) Peces invasores en el noroeste de México. In: Mendoza R, Koleff P (eds) Especies acuáticas invasoras en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, pp 375–399
Sada DW, Vinyard GL (2002) Anthropogenic changes in historical biogeography of Great Basin aquatic biota. In: Hershler R, Madsen DB, Currey DR (eds) Great Basin aquatic systems history, vol 33. Smithsonian Contributions to the Earth Sciences, Washington, pp 227–293
Sada DW, Fleishman E, Murphy DD (2005) Associations among spring-dependent aquatic assemblages and environmental and land use gradients in a Mojave Desert mountain range. Divers Distrib 11:91–99
Seager R, Ting M, Held I, Kushnir Y, Lu J, Vecchi G, Huang H-P, Harnik N, Leetmaa A, Lau N-C, Li C, Velez J, Naik N (2007) Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316:1181–1184
Shepard WD (1993) Desert springs- both rare and endangered. Aquat Conserv Mar Freshw Ecosyst 3:351–359
Stevens LE, Meretsky VJ (eds) (2008) Aridland springs in North America: ecology and conservation. Arizona-Sonora Desert museum studies in natural history. University of Arizona Press, Tucson
Suhling F, Sahlen G, Martens A, Marais E, Schutte C (2006) Dragonfly assemblages in arid tropical environments. Biodivers Conserv 15:311–332
Townsend CR (1996) Invasion biology and ecological impacts of brown trout, Salmo trutta, in New Zealand. Biol Conserv 78:13–22
Unmack PJ, Minckley WL (2008) The demise of desert springs. In: Stevens LE, Meretsky VJ (eds) Aridland springs in North America: ecology and conservation. Arizona-Sonora Desert museum studies in natural history. University of Arizona Press, Tucson, pp 11–34
Vitule JRS, Freire CA, Simberloff D (2009) Introduction of non-native fish can certainly be bad. Fish Fish 10:98–108
Acknowledgments
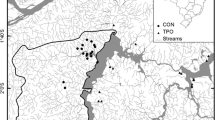
This work was made possible in part by two grants from the non-profit group T&E Inc., and is dedicated to the memory of the group’s founder, Tom Wootten. Many thanks to Enrique Castillo-Grijalva for facilitating access to several survey sites and accompanying us on several site visits, and to Doña Olga Armenta, Miguel Velázquez-Armenta, Ramón Villafraña, and Delfín Magallanes-Molina for allowing access to various study sites. Thanks to Claire Zugmeyer for providing the digital elevation map used to produce Fig. 1. Thanks to Dave Ruiter (Trichoptera), Kelly Miller (Dytiscidae), and Bill Shepard and Cheryl Barr (Elmidae) for taxonomic help with adult aquatic insects during the course of this project. Thanks to Jani Heino and two anonymous referees for their helpful comments on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jani Heino.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Bogan, M.T., Noriega-Felix, N., Vidal-Aguilar, S.L. et al. Biogeography and conservation of aquatic fauna in spring-fed tropical canyons of the southern Sonoran Desert, Mexico. Biodivers Conserv 23, 2705–2748 (2014). https://doi.org/10.1007/s10531-014-0745-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-014-0745-z