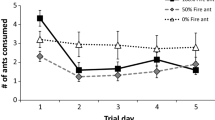
Abstract
Contemporary adaptation of native prey species to invasive predators has been relatively well documented, but that of native predators to invasive prey has received less attention. Because the level of impact an invasive species will have on its predators versus its prey will determine changes in community trophic structure, it is important to understand how native predators respond to novel prey. Here we examine the response of native fence lizards to the invasion of red imported fire ants, a novel toxic prey. Examining invaded and uninvaded lizard populations, we tested whether or not aversion-learning occurs in juvenile fence lizards over successive feedings (within lifetime), how previous fire ant exposure may affect avoidance behavior (over generations), and whether population differences are consistent when prey choice exists. We also examine rates of phenotypic divergence in traits associated with the native species as both predator and prey. Aversion-learning did not occur in either population. Instead, the incidence of fire ant consumption increased over both successive feedings and generations. Lizards from the fire ant invaded population had a higher propensity to eat fire ants than fire ant-naïve lizards, even when given a choice between prey items. We found greater phenotypic divergence in traits associated with the native species as predator on, versus as prey to, fire ants. Although the strategy of eating these novel toxic prey can impose survival costs in the short term, over the longer-term, eating fire ants may cost little or even benefit survivors.



Similar content being viewed by others
References
Boronow KE, Langkilde T (2010) Sublethal effects of invasive fire ant venom on a native lizard. J Exp Zool 313A:17–23
Bulte G, Blouin-Demers G (2008) Northern map turtles (Graptemys geographica) derive energy from the pelagic pathway through predation on zebra mussels (Dreissena polymorpha). Freshwat Biol 53:497–508
Burghardt G, Wilcoxon H, Czaplicki J (1973) Conditioning in garter snakes: aversion to palatable prey induced by delayed illness. Anim Learn Behav 1:317–320
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer, New York
Callcott AA, Collins HL (1996) Invasion and range expansion of imported fire ants Hymenoptera: formicidae in North America from 1918 to 1995. Fla Entomol 79:240–251
Carlsson NOL, Sarnelle O, Strayer DL (2009) Native predators and exotic prey: an acquired taste? Front Ecol Environ 7:525–532
Carroll SP (2007) Natives adapting to invasive species: ecology, genes, and the sustainability of conservation. Ecol Res 22:892–901
Carroll SP, Klassen SP, Dingle H (1998) Rapidly evolving adaptations to host ecology and nutrition in the soapberry bug. Evol Ecol 12:955–968
Code of Federal Regulations (2012) Title 7. Part 301.81. Subpart: imported fire ant. In. http://ecfr.gpoaccess.gov
Crossland MR (2001) Ability of predatory native Australian fishes to learn to avoid toxic larvae of the introduced toad Bufo marinus. J Fish Biol 59:319–329
Dawkins R, Krebs JR (1979) Arms races between and within species. Proc R Soc Lond Ser B Biol Sci 205:489–511
Demarco VG, Ferguson GW (1985) Maximum prey size of an insectivorous lizard, Sceloporus undulatus garmani. Copeia 1985:1077–1080
Freidenfelds NA, Robbins TR, Langkilde T (2012) Evading invaders: the effectiveness of a behavioral response acquired through lifetime exposure. Behav Ecol 23:659–664
Gill AB (2003) The dynamics of prey choice in fish: the importance of prey size and satiation. J Fish Biol 63:105–116
Greenlees MJ, Phillips BL, Shine R (2010) Adjusting to a toxic invader: native Australian frogs learn not to prey on cane toads. Behav Ecol 21:966–971
Hendry AP, Kinnison MT (1999) Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution 53:1637–1653
Hutchinson DA, Mori A, Savitzky AH et al (2007) Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus. Proc Nat Acad Sci USA 104:2265–2270
King JR, Tschinkel WR (2008) Experimental evidence that human impacts drive fire ant invasions and ecological change. Proc Nat Acad Sci USA 105:20339–20343
King RB, Ray JM, Stanford KM (2006) Gorging on gobies: beneficial effects of alien prey on a threatened vertebrate. Can J Zool 84:108–115
Langkilde T (2009a) Holding ground in the face of invasion: native fence lizards (Sceloporus undulatus) do not alter their habitat use in response to introduced fire ants (Solenopsis invicta). Can J Zool 87:626–634
Langkilde T (2009b) Invasive fire ants alter behavior and morphology of native lizards. Ecology 90:208–217
Langkilde T (2010) Repeated exposure and handling effects on the escape response of fence lizards to encounters with invasive fire ants. Anim Behav 79:291–298
Langkilde T, Freidenfelds NA (2010) Consequences of envenomation: red imported fire ants have delayed effects on survival but not growth of native fence lizards. Wildl Res 37:566–573
Leache AD (2009) Species tree discordance traces to phylogeographic clade boundaries in North American fence lizards (Sceloporus). Syst Biol 58:547–559
Magoulick DD, Lewis LC (2002) Predation on exotic zebra mussels by native fishes: effects on predator and prey. Freshw Biol 47:1908–1918
Molloy DP, Powell J, Ambrose P (1994) Short-term reduction of adult zebra mussels (Dreissena polymorpha) in the Hudsun River near Catskill, New York: an effect of juvenile blue crab (Callinectes sapidus) predation. J Shellfish Res 13:367–371
Nelson DWM, Crossland MR, Shine R (2011) Foraging responses of predators to novel toxic prey: effects of predator learning and relative prey abundance. Oikos 120:152–158
Pan W (2001) Akaike’s information criterion in generalized estimating equations. Biometrics 57:120–125
Parker WS (1994) Demography of the fence lizard, Sceloporus undulatus, in northern Mississippi. Copeia 1994:136–152
Partridge ME, Blackwood W, Hamilton RG et al (2008) Prevalence of allergic sensitization to imported fire ants in children living in an endemic region of the southeastern United States. Ann Allergy Asthma Immunol 100:54–58
Petrie SA, Knapton RW (1999) Rapid increase and subsequent decline of zebra and quagga mussels in Long Point Bay, Lake Erie: possible influence of waterfowl predation. J Great Lakes Res 25:772–782
Phillips BL, Shine R (2006) An invasive species induces rapid adaptive change in a native predator: cane toads and black snakes in Australia. Proc R Soc B Biol Sci 273:1545–1550
Porter SD, Savignano DA (1990) Invasion of polygene fire ants decimate native ants and disrupt arthropod community. Ecology 71:2095–2106
Ricciardi A (2001) Facilitative interactions among aquatic invaders: is an “invasional meltdown” occurring in the Great Lakes? Can J Fish Aquat Sci 58:2513–2525
Ricciardi A, MacIsaac HJ (2000) Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends Ecol Evol 15:62–65
Robbins TR, Langkilde T (2012) The consequences of lifetime and evolutionary exposure to toxic prey: changes in avoidance behavior through ontogeny. J Evol Biol. doi:10.1111/j.1420-9101.2012.02583.x
Rozin P, Schiller D (1980) The nature and aquisition of a preference for chili pepper by humans. Motiv Emotion 4:77–101
Rozin P, Gruss L, Berk G (1979) Reversal of innate aversions: attempts to induce a preference for chili peppers in rats. J Comp Physiol Psychol 93:1001–1014
Somaweera R, Webb JK, Brown GP et al (2011) Hatchling Australian freshwater crocodiles rapidly learn to avoid toxic invasive cane toads. Behaviour 148:501–517
SPSS for Windows R (2011). SPSS Inc., Chicago
Stewart TW, Miner JG, Lowe RL (1998) Macroinvertebrate communities on hard substrates in western Lake Erie: structuring effects of Dreissena. J Great Lakes Res 24:868–879
Suarez AV, Case TJ (2002) Bottom-up effects on persistence of a specialist predator : ant invasions and horned lizards. Ecol Appl 12:291–298
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing., Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Tschinkel WR (2006) The fire ants. Belknap/Harvard University Press, Cambridge, MA
Webb SL, Henke S (2003) Defensive strategies of Texas horned lizards (Phrynosoma cornutum) against red imported fire ants. Herpetol Rev 34:327–328
Webb JK, Brown GP, Child T et al (2008) A native dasyurid predator (common planigale, Planigale maculata) rapidly learns to avoid a toxic invader. Austral Ecol 33:821–829
Acknowledgments
We thank B. Chitterling, one anonymous reviewer, and D. Simberloff for comments and suggestions that greatly improved this manuscript. We thank B. Carlson for help with statistical analyses in R and the personnel at Solon Dixon Forestry and Education Center and Saint Francis National Forest for logistical support. We also extend thanks to the Landsdale family for access to their property. The research presented here adhered to Guidelines for the Use of Animals in Research, and the Institutional Guidelines of Pennsylvania State University. Animal collection was authorized by the respective State’s Permits. This research was funded by the National Science Foundation (DEB- 0949483) to TL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robbins, T.R., Freidenfelds, N.A. & Langkilde, T. Native predator eats invasive toxic prey: evidence for increased incidence of consumption rather than aversion-learning. Biol Invasions 15, 407–415 (2013). https://doi.org/10.1007/s10530-012-0295-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0295-9