Abstract
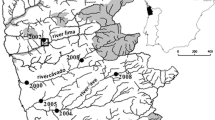
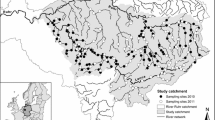
In this study we use a combination of techniques to assess the general risk of invasion by the aquatic invader Dikerogammarus villosus in Great Britain at multiple scales. First, bioclimatic models (Support Vector Machine algorithm) were used to identify regions showing the highest climatic match with the European range of the species, and that might be at highest risk of initial colonization if provided with propagules. The model showed that nearly 60% of Great Britain shows the minimum bioclimatic suitability for the species, particularly southern and eastern England. Afterwards, a Network Analysis was used to model the natural spatio-temporal spread of the killer shrimp in the Great Ouse River catchment (SE England), the first region invaded by this species. This model suggested that the northern part of the catchment, including two relevant Ramsar sites (Ouse Washes and Wicken fen) are at serious risk of being invaded in the short-term (<5 years). Taking into account the rapid spread of the killer shrimp in other European countries, its broad environmental tolerance, the high climate suitability of broad areas of Great Britain to the species, the current spread of other Ponto-Caspian species, and the high natural and artificial connectivity of the hydrological network, we conclude that D. villosus is very likely to continue its spread in Great Britain, dramatically affecting the native biodiversity. The multi-scale approach proposed in this study combines large-scale bioclimatic models with local-scale dispersal models, providing managers with a powerful spatial and temporal basis for informed decision-making.



Similar content being viewed by others
References
Aldridge DC (2005) The zebra mussel, Dreissena polymorpha, in the Anglian region: results of river and water treatment works surveys. Cambridge Environmental Consultants, Cambridge, p 31
Aldridge DC (2010) Dreissena polymorpha in Great Britain: history of spread, impacts and control. In: van der Velde G, Rajagopal S, bij de Vaate A (eds) The Zebra Mussel in Europe. Backhuys Publishers, Leiden, pp 79–91
Aldridge DC, Elliott P, Moggridge GD (2004) The recent and rapid spread of the zebra mussel (Dreissena polymorpha) in Great Britain. Biol Conserv 119:253–261
Beaumont LJ, Gallagher RV, Thuiller W et al (2009) Different climatic envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers Distrib 15:409–420
Bilton DT, Freeland JR, Okamura B (2001) Dispersal in freshwater invertebrates. Annu Rev Ecol Syst 32:159–181
Boets P, Lock K, Messiaen M et al (2010) Combining data-driven methods and lab studies to analyse the ecology of Dikerogammarus villosus. Ecol Inform 5:133–139
Bruijs MCM, Kelleher B, van der Velde G et al (2001) Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: indicators of further dispersal via ballast water transport. Arch Hydrobiol 152:633–646
Casellato S, Visentin A, La Piana G (2007) The predatory impact of Dikerogammarus villosus on fish. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution and threats. Springer, Dordrecht, pp 495–506
Chen PF, Wiley EO, Mcnyset KM (2007) Ecological niche modeling as a predictive tool: silver and bighead carps in North America. Biol Invasions 9:43–51
de Souza Muñoz M, De Giovanni R, de Siqueira M, Sutton T, Brewer P, Pereira R, Canhos D, Canhos V (2011) openModeller: a generic approach to species’ potential distribution modelling. GeoInformatica 15:111–135
Devin S, Beisel JN, Bachmann V et al (2001) Dikerogammarus villosus (Amphipoda: Gammaridae): another invasive species newly established in the Moselle river and French hydrosystems. Ann De Limnol Int J Limnol 37:21–27
Devin S, Piscart C, Beisel JN et al (2003) Ecological traits of the amphipod invader Dikerogammarus villosus on a mesohabitat scale. Arch Hydrobiol 158:43–56
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084
Drake JM, Bossenbroek JM (2004) The potential distribution of zebra mussels in the United States. Bioscience 54:931–941
Drake JM, Randin C, Guisan A (2006) Modelling ecological niches with support vector machines. J Appl Ecol 43:424–432
Elith J, Graham CH (2009) Do they? how do they? why do they differ? On finding reasons for differing performances of species distribution models. Ecography 32:66–77
Elliott JM (2002) The drift distances and time spent in the drift by freshwater shrimps, Gammarus pulex, in a small stony stream, and their implications for the interpretation of downstream dispersal. Freshw Biol 47:1403–1417
Elliott JM (2003) A comparative study of the dispersal of 10 species of stream invertebrates. Freshw Biol 48:1652–1668
Gergs R, Rothhaupt KO (2008) Feeding rates, assimilation efficiencies and growth of two amphipod species on biodeposited material from zebra mussels. Freshw Biol 53:2494–2503
Gherardi F (2007) Biological invasions in inland waters: an overview. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Springer, Netherlands, pp 3–25
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Hancock MA, Hughes JM (1999) Direct measures of instream movement in a freshwater shrimp using a genetic marker. Hydrobiologia 416:23–32
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1:29–36
Hijmans RJ, Cameron SE, Parra JL et al (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Jocque M, Field R, Brendonck L et al (2010) Climatic control of dispersal-ecological specialization trade-offs: a metacommunity process at the heart of the latitudinal diversity gradient? Global Ecol Biogeogr 19:244–252
Keller RP, Ermgassen P, Aldridge DC (2009) Vectors and timing of freshwater invasions in Great Britain. Conserv Biol 23:1526–1534
Kraft CE, Sullivan PJ, Karatayev AY et al (2002) Landscape patterns of an aquatic invader: assessing dispersal extent from spatial distributions. Ecol Appl 12:749–759
Kumar S, Spaulding SA, Stohlgren TJ et al (2009) Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front Ecol Environ 7:415–420
Leathwick JR, Elith J, Chadderton WL et al (2008) Dispersal, disturbance and the contrasting biogeographies of New Zealand’s diadromous and non-diadromous fish species. J Biogeogr 35:1481–1497
Leuven R, van der Velde G, Baijens I et al (2009) The River Rhine: a global highway for dispersal of aquatic invasive species. Biol Invasions 11:1989–2008
Loo SE, Mac Nally R, Lake PS (2007) Forecasting New Zealand mudsnail invasion range: model comparisons using native and invaded ranges. Ecol Appl 17:181–189
MacNeil C, Platvoet D, Dick JTA et al (2010) The Ponto-Caspian ‘killer shrimp’, Dikerogammarus villosus (Sowinsky, 1894), invades the British Isles. Aquat Invasions 5:441–445
Madgwick G, Aldridge DC (2011) Killer shrimps in Britain: hype or horror? British Wildlife Publishing, Dorset, UK, pp 408–412
McNyset KM (2005) Use of ecological niche modelling to predict distributions of freshwater fish species in Kansas. Ecol Freshw Fish 14:243–255
Niggebrugge K, Durance I, Watson AM et al (2007) Applying landscape ecology to conservation biology: spatially explicit analysis reveals dispersal limits on threatened wetland gastropods. Biol Conserv 139:286–296
Oreska M, Aldridge D (2011) Estimating the financial costs of freshwater invasive species in Great Britain: a standardized approach to invasive species costing. Biol Invasions 13:305–319
Peterson AT (2003) Predicting the geography of species’ invasions via ecological niche modeling. Q Rev Biol 78:419–433
Phillips SJ, Elith J (2010) POC plots: calibrating species distribution models with presence-only data. Ecology 91:2476–2484
Piscart C, Devin S, Beisel JN et al (2003) Growth-related life-history traits of an invasive gammarid species: evaluation with a Laird-Gompertz model. Can J Zool Revue Canadienne De Zoologie 81:2006–2014
Platt JC (1999) Probabilistic outputs for Support Vector Machines and comparison to regularized likelihood methods. In: Scholkopf B, Burges CJC, Smola AJ (eds) Advances in Kernel methods—support vector learning. MIT Press, Cambridge, pp 185–208
Platvoet D, Dick JTA, MacNeil C et al (2009) Invader-invader interactions in relation to environmental heterogeneity leads to zonation of two invasive amphipods, Dikerogammarus villosus (Sowinsky) and Gammarus tigrinus. Biol Invasions 11:2085–2093
Pockl M (2009) Success of the invasive Ponto-Caspian amphipod Dikerogammarus villosus by life history traits and reproductive capacity. Biol Invasions 11:2021–2041
Pockl M, Webb BW, Sutcliffe DW (2003) Life history and reproductive capacity of Gammarus fossarum and G. roeseli (Crustacea: Amphipoda) under naturally fluctuating water temperatures: a simulation study. Freshw Biol 48:53–66
Ricciardi A, Rasmussen JB (1998) Predicting the identity and impact of future biological invaders: a priority for aquatic resource management. Can J Fish Aquat Sci 55:1759–1765
Rodda GH, Jarnevich CS, Reed RN (2011) Challenges in identifying sites climatically matched to the native ranges of animal invaders. Plos One 6(2):e14670. doi:10.1371/journal.pone.0014670
Sala OE, Chapin FS, Armesto JJ et al (2000) Biodiversity—global biodiversity scenarios for the year 2100. Science 287:1770–1774
Schick RS, Lindley ST (2007) Directed connectivity among fish populations in a riverine network. J Appl Ecol 44:1116–1126
Schölkopf B, Smola AJ, Williamson RC et al (2000) New support vector algorithms. Neural Comput 12:1207–1245
Sheader M (1996) Factors influencing egg size in the gammarid amphipod Gammarus insensibilis. Mar Biol 124:519–526
Timar L, Phaneuf DJ (2009) Modeling the human-induced spread of an aquatic invasive: the case of the zebra mussel. Ecol Econ 68:3060–3071
Urban DL, Minor ES, Treml EA et al (2009) Graph models of habitat mosaics. Ecol Lett 12:260–273
Václavík T, Meentemeyer RK (2011) Equilibrium or not? Modelling potential distribution of invasive species in different stages of invasion. Divers Distrib. doi:10.1111/j.1472-4642.2011.00854.x
van Riel MC, van der Velde G, Rajagopal S et al (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58
Wiens JA (2002) Riverine landscapes: taking landscape ecology into the water. Freshw Biol 47:501–515
Wijnhoven S, van Riel MC, van der Velde G (2003) Exotic and indigenous freshwater gammarid species: physiological tolerance to water temperature in relation to ionic content of the water. Aquat Ecol 37:151–158
Acknowledgments
The research leading to these results has received funding from the European Commission (FP7/2007-2013, Marie Curie IEF program) under grant agreement no 251785. The author would like to thank C. McLaughlan for her help with the English editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallardo, B., Errea, M.P. & Aldridge, D.C. Application of bioclimatic models coupled with network analysis for risk assessment of the killer shrimp, Dikerogammarus villosus, in Great Britain. Biol Invasions 14, 1265–1278 (2012). https://doi.org/10.1007/s10530-011-0154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-011-0154-0