Abstract
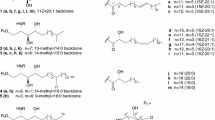
Glucosylceramide and galactosylceramide were detected in three Aspergillus species: Aspergillus oryzae, Aspergillus sojae and Aspergillus. awamori, using borate-coated TLC. The cerebrosides from A. oryzae were further purified by ion exchange and iatrobeads column chromatographies with or without borate, and determined the composition of sugar, fatty acid and sphingoid base by GC/MS, MALDI-TOF/MS and 1H-NMR. We identified them as β-glucosylceramide and β-galactosylceramide. The ceramide moiety of both cerebrosides consisted mainly of 2-hydroxystearic acid and either 9-methyl-octadeca-4, 8-sphingadienine or octadeca-4, 8-sphingadienine. To our knowledge, this is the first study to provide evidence for the presence of β-galactosylceramide in A. oryzae.




Similar content being viewed by others
References
Alessandrini F, Pfister S, Kremmer E, Gerber JK, Ring J, Behrendt H (2004) Alterations of glucosylceramide-β-glucosidase levels in the skin of patients with psoriasis vulgaris. J Invest Dermatol 123:1030–1036
Aoki K, Uchiyama R, Yamauchi S, Katayama T, Itonori S, Sugita M, Hada N, Yamada-Hada J, Takeda T, Kumagai H, Yamamoto K (2004) Newly discovered neutral glycosphingolipids in aureobasidin A-resistant zygomycetes: identification of a novel family of gala-series glycolipids with core Galα1-6Galβ1-6Galβ sequences. J Biol Chem 279:32028–32034
Barreto-Bergter E, Sassaki GL, de Souza LM (2011) Structural analysis of fungal cerebrosides. Front Microbiol 2:239
Boas MH, Egge H, Pohlentz G, Hartmann R, Bergter EB (1994) Structural determination of N-2′-hydroxyoctadecenoyl-1-O-β-D-glucopyranosyl-9-methyl-4, 8-sphingadienine from species of Aspergillus. Chem Phys Lipids 70:11–19
Fujino Y, Ohnishi M (1977) Structure of cerebroside in Aspergillus oryzae. Biochim Biophys Acta 486:161–171
Hara M, Uchida Y, Haratake A, Mimura K, Hamanaka S (1998) Galactocerebroside and not glucocerebroside or ceramide stimulate epidermal β-glucocerebrosidase activity. J Dermatol Sci 16:111–119
Hata Y, Ishida H, Ichikawa E, Kawato A, Suginami K, Imayasu S (1998) Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene 207:127–134
Hirata M, Tsuge K, Jayakody LN, Urano Y, Sawada K, Inaba S, Nagao K, Kitagaki H (2012) Structural determination of glucosylceramides in the distillation remnants of shochu, the Japanese traditional liquor, and its production by Aspergillus kawachii. J Agric Food Chem 60:11473–11482
Kawai G, Ikeda Y (1985) Structure of biologically active and inactive cerebrosides prepared from Schizophyllum commune. J Lipid Res 26:338–343
Kean EL (1966) Separation of gluco-and galactocerebrosides by means of borate thin-layer chromatography. J Lipid Res 7:449–452
Levery SB, Momany M, Lindsey R, Toledo MS, Shayman JA, Fuller M, Brooks K, Doong RL, Straus AH, Takahashi HK (2002) Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett 525:59–64
Lim BC, Kim HJ, Oh DK (2007) High production of d-tagatose by the addition of boric acid. Biotechnol Prog 23:824–828
Mrochek JE, Dinsmore SR, Waalkes TP (1975) Liquid-chromatographic analysis for neutral carbohydrates in serum glycoproteins. Clin Chem 21:1314–1322
Nimrichter L, Rodrigues ML (2011) Fungal glucosylceramides: from structural components to biologically active targets of new antimicrobials. Front Microbiol 2:212
Ramamoorthy V, Cahoon EB, Li J, Thokala M, Minto RE, Shah DM (2007) Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum. Mol Microbiol 66:771–786
Rittenour WR, Chen M, Cahoon EB, Harris SD (2011) Control of glucosylceramide production and morphogenesis by the Bar1 ceramide synthase in Fusarium graminearum. PLoS ONE 6:e19385
Tani Y, Funatsu T, Ashida H, Ito M, Itonori S, Sugita M, Yamamoto K (2010) Novel neogala-series glycosphingolipids with terminal mannose and glucose residues from Hirsutella rhossiliensis, an aureobasidin A-resistant ascomycete fungus. Glycobiology 20:433–441
Tani Y, Nakamura K, Sawa R, Nishio M, Saito S, Ito M, Itonori S, Mihara H (2013) Novel neogala-series glycosphingolipids with a terminal glucose residue from the fungus Mariannaea elegans. Biosci Biotechnol Biochem 77:754–759
Zhang Y, Wang S, Li XM, Cui CM, Feng C, Wang BG (2007) New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids 42:759–764
Acknowledgments
We thank Dr. N. Hada (Keio University) for help with NMR analyses, and Messrs. M. Nishio and H. Kojima (Ritsumeikan University) for technical assistance with MALDI-TOF/MS and NMR analyses. This work was supported in part by a Grant-in-Aid for Scientific Research (B 16380063, to K.Y.) from the Japan Society for the Promotion of Science; and Y.T. was supported by the 21st Century Center of Excellence (COE) program of the Ministry of Education, Culture, Sports, Science, and Technology, Japan to the Graduate School of Biostudies and Institute for Virus Research, Kyoto University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tani, Y., Amaishi, Y., Funatsu, T. et al. Structural analysis of cerebrosides from Aspergillus fungi: the existence of galactosylceramide in A. oryzae . Biotechnol Lett 36, 2507–2513 (2014). https://doi.org/10.1007/s10529-014-1631-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1631-1