Abstract
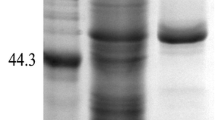
Neutral protease I from Aspergillus oryzae 3.042 was expressed in Pichia pastoris and its N-glycosylation properties were analyzed. After purification by nickel-affinity chromatography column, the recombinant neutral protease (rNPI) was confirmed to be N-glycosylated by periodicacid/Schiff’s base staining and Endo H digestion. Moreover, the deglycosylated protein’s molecular weight decreased to 43.3 kDa from 54.5 kDa analyzed by SDS-PAGE and MALDI–TOF–MS, and the hyperglycosylation extent was 21 %. The N-glycosylation site of rNPI was analyzed by nano LC–MS/MS after digesting by trypsin and Glu-C, and the unique potential site Asn41 of mature peptide was found to be glycosylated. Homology modeling of the 3D structure of rNPI indicated that the attached N-glycans hardly affected neutral protease’s activity due to the great distance away from the active site of the enzyme.





Similar content being viewed by others
References
Arnold K, Bordoli L, Kopp J et al (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201
Carlsson SR (1993) Isolation and characterization of glycoproteins. In: Fukuda M, Kobata A (eds) Glycobiology, a practical approach. Oxford University Press Inc., New York, pp 1–26
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay—casein as a substrate. J Vis Exp 19:899–900
De Pourcq K, De Schutter K, Callewaert N et al (2010) Engineering of glycosylation in yeast and other fungi: current state and perspectives. Appl Microbiol Biotechnol 87:1617–1631
Dean N (1999) Asparagine-linked glycosylation in the yeast golgi. BBA-Gen Subj 1426:309–322
Gomis-Rüth FX, Botelho TO, Bode W (2012) A standard orientation for metallopeptidases. BBA-Proteins Proteomics 1824:157–163
Grinna LS, Tschopp JF (1989) Size distribution and general structural features of N-linked oligosaccharides from the methylotrophic yeast, Pichia pastoris. Yeast 5:107–115
Guo M, Hang H, Zhu T et al (2008) Effect of glycosylation on biochemical characterization of recombinant phytase expressed in Pichia pastoris. Enzym Microb Technol 42:340–345
Hsiao ES, Chen JC, Tsai HY et al (2009) Determination of N-glycosylation site and glycan structures of pectin methylesterase in jelly fig (Ficus awkeotsang) achenes. J Agric Food Chem 57:6757–6763
Ke Y, Huang WQ, Li JZ et al (2012) Enzymatic characteristics of a recombinant neutral protease I (rNpI) from Aspergillus oryzae expressed in Pichia pastoris. J Agric Food Chem 60:12164–12169
Machida M, Asai K, Sano M et al (2005) Genome sequencing and analysis of Aspergillus oryzae. Nature 438:1157–1161
Montesino R, Garcia R, Quintero O et al (1998) Variation in N-linked oligosaccharide structures on heterologous proteins secreted by the methylotrophic yeast Pichia pastoris. Protein Expr Purif 14:197–207
Tatsumi H, Murakami S, Tsuji R et al (1991) Cloning and expression in yeast of a cDNA clone encoding Aspergillus oryzae neutral protease II, a unique metalloprotease. Mol Gen Genet 228:97–103
Wang LH, Li DQ, Fu Y et al (2007) pFind 2.0: a software package for peptide and protein identification via tandem mass spectrometry. Rapid Commun Mass Spectrom 21:2985–2991
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40
Acknowledgments
This work has been supported by the National Natural Science Foundation of P. R. China (Project No. 31260389).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lei, D., Xu, Y., He, Q. et al. Glycosylation analysis of recombinant neutral protease I from Aspergillus oryzae expressed in Pichia pastoris . Biotechnol Lett 35, 2121–2127 (2013). https://doi.org/10.1007/s10529-013-1314-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1314-3