Abstract
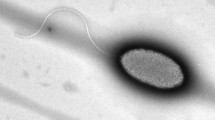
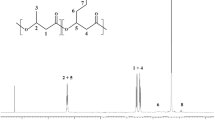
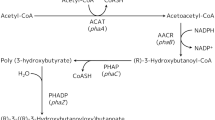
The class III poly(hydroxyalkanoate) synthase (PHAS) genes (phaC and phaE) of a photosynthetic bacterium, Allochromatium vinosum ATCC 35206, were cloned, sequenced and expressed in a heterologous host. PCR coupled with a chromosomal gene-walking method was used to clone and subsequently sequence the contiguous phaC (1,068 bps) and phaE (1,065 bps) genes of A. vinosum ATCC 35206. BLASTP search of protein databases showed that the gene-products of phaC and phaE are different (<66% identities) from the previously reported class III PHASs such as those of A. vinosum DSM180. Domain analysis revealed the presence of a conserved α/β-hydrolase fold in PhaC, the putative gene-product of phaC. Upon electroporation of a poly(hydroxybutanoate) (PHB)-negative mutant of Ralstonia eutropha PHB−4 with a shuttle plasmid pBHR1 containing the newly cloned phaC and phaE genes, the bacteria resumed the synthesis of PHB, albeit at a low level (4–5% of the cell dry wt) due to kanamycin selection pressure. We further showed that the recombinant strain grown in kanamycin-containing culture medium synthesized a blend of PHA that also contains a high content of 3-hydroxyoctanoate and 3-hydroxydecanoate as its repeat-unit monomers. Genomic analysis suggested the existence of two PHA synthase genes in R. eutropha. The results of this study not only make available a phylogenetically diverse type III phaC and phaE genes, but also confirm through kanamycin selection pressure the existence of multiple PHA biosynthesis systems in R. eutropha.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ashby RD, Solaiman DKY, Foglia TA (2002) The synthesis of short- and medium-chain-length poly(hydroxyalkanoate) mixtures from glucose- or alkanoic acid-grown Pseudomonas oleovorans. J Ind Microbiol Biotechnol 28:147–153
Chen GQ, Wu Q (2005) The applications of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
de Andrade Rodrigues MF, Vicente EJ, Steinbüchel A (2000) Studies on polyhydroxyalkanoate (PHA) accumulation in a PHA synthase I-negative mutant of Burkholderia cepacia generated by homogenotization. FEMS Microbiol Lett 193:179–185
Fritzsche K, Lenz RW, Fuller RC (1999) Bacterial polyesters containing branched poly(beta-hydroxyalkanoate) units. Int J Biol Macromol 12:92–101
Gerngross TU, Snell KD, Peoples OP, Sinckey AJ, Cushai E, Masamune S, Stubbe J (1994) Overexpression and purification of the soluble polyhydroxyalkanoate synthase from Alcaligenes eutrophus: evidence for a required posttranslational modification for catalytic activity. Biochemistry 33:9311–9320
Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100:965–973
Hai T, Hein S, Steinbüchel A (2001) Multiple evidence for widespread and general occurrence of type-III PHA synthases in cyanobacteria and molecular characterization of the PHA synthases from two thermophilic cyanobacteria: Chlorogloeopsis fritschii PCC 6912 and Synechococcus sp. strain MA19. Microbiology 147:3047–3060
Hai T, Lange D, Rabus R, Steinbüchel A (2004) Polyhydroxyalkanoate (PHA) accumulation in sulfate-reducing bacteria and identification of a class III PHA synthase (phaEC) in Desulfococcus multivorans. Appl Environ Microbiol 70:4440–4448
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hein S, Tran H, Steinbüchel A (1998) Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch Microbiol 170:162–170
Kim YB, Lenz RW, Fuller RC (1992) Poly(β-hydroxyalkanoate) copolymers containing brominated repeating units produced by Pseudomonas oleovorans. Macromolecules 25:1852–1857
Kim O, Gross RA, Rutherford DR (1995) Bioengineering of poly(β-hydroxyalkanoates) for advanced material applications: incorporation of cyano and nitrophenoxy side chain substituents. Can J Microbiol 41(suppl 1):32–43
Liebergesell M, Steinbüchel A (1992) Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem 209:135–150
Liebergesell M, Steinbüchel A (1993) Cloning and molecular analysis of the poly(3-hydroxybutyric acid) biosynthetic genes of Thiocystis violacea. Appl Microbiol Biotechnol 38:493–501
Liebergesell M, Mayer F, Steinbüchel A (1993) Analysis of polyhydroxyalkanoic acid-biosynthesis genes of anoxygenic phototrophic bacteria reveals synthesis of a polyester exhibiting an unusual composition. Appl Microbiol Biotechnol 40:292–300
Lu Q, Han J, Zhou L, Zhou J, Xiang H (2008) Genetic and biochemical characterization of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) synthase in Haloferax mediterranei. J Bacteriol 190:4173–4180
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydoxyalkanoates): from dna to plastic. Microbiol Mol Biol Rev 63:21–53
Pieper U, Steinbüchel A (1992) Identification, cloning and sequence analysis of the poly(3-hydroxyalkanoic acid) synthase gene of the Gram-positive bacterium Rhodococcus ruber. FEMS Microbiol Lett 96:73–80
Pohlmann A, Fricke WF, Reinecke F, Kusian B, Liesegang H, Cramm R, Eitinger T, Ewering C, Pötter M, Schwartz E, Strittmatter A, Voss I, Gottschalk G, Steinbüchel A, Friedrich B, Bowien B (2006) Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat Biotechnol 24:1257–1262
Reese MG (2001) Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem 26:51–56
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Rehm BHA, Steinbüchel A (1999) Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol 25:3–19
Schlegel HG, Lafferty R, Krauss I (1970) The isolation of mutants not accumulating poly-β-hydroxybutyric acid. Arch Microbiol 71:283–294
Solaiman DKY (1998) Genetic transformation of Pseudomonas oleovorans by electroporation. Biotechnol Tech 12:829–832
Solaiman DKY (2003) Biosynthesis of medium-chain-length poly(hydroxyalkanoates) with altered composition by mutant hybrid PHA synthases. J Ind Microbiol Biotechnol 30:322–326
Solaiman DKY, Ashby RD (2005) Rapid genetic characterization of poly(hydroxyalkanoate) synthase and its applications. Biomacromolecules 6:532–537
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN (2006) Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl Microbiol Biotechnol 71:783–789
Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbüchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol 171:73–80
Stubbe J, Tian J, He A, Sinskey AJ, Lawrence AG, Liu P (2005) Nontemplate-dependent polymerization processes: polyhydroxyalkanoate synthases as a paradigm. Annu Rev Biochem 74:433–480
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Yuan W, Jia Y, Tian J, Snell KD, Muh U, Sinskey AJ, Lambalot RH, Walsh CT, Stubbe J (2001) Class I and III polyhydroxyalkanoate synthases from Ralstonia eutropha and Allochromatium vinosum: characterization and substrate specificity studies. Arch Biochem Biophys 394:87–98
Acknowledgments
The authors thank Nicole Crocker and Marshall Reed for excellent technical assistance. The support of Dr. Peter Cooke on advanced microscopic imaging and Dr. David Needleman on DNA sequence determination is recognized. Thanks are also due Professor Yong-Hyun Lee (Kyungpook National University, Daegu, Republic of Korea) for providing pBHR1 plasmid. This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2003-35504-13751.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Aneja, K.K., Ashby, R.D. & Solaiman, D.K.Y. Altered composition of Ralstonia eutropha poly(hydroxyalkanoate) through expression of PHA synthase from Allochromatium vinosum ATCC 35206. Biotechnol Lett 31, 1601–1612 (2009). https://doi.org/10.1007/s10529-009-0052-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0052-z